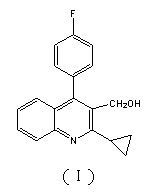
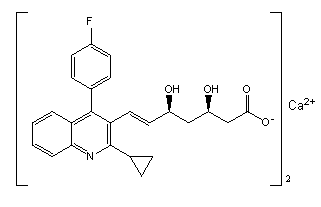
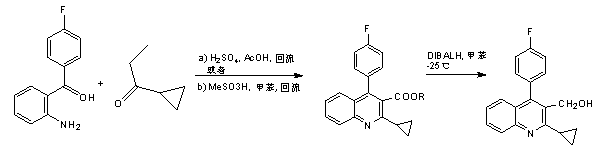
Preparation method of pitavastatin calcium intermediate compound
A system and compound technology, applied in the direction of organic chemistry, can solve the problems of low reaction yield, expensive DIBAL-H, unfavorable industrial production, etc., and achieve the effect of reducing production cost and improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] step 1:
[0028]
[0029] In a 250ml three-neck flask, add 18.2g of the compound of formula (II-1) (HPLC: 99.5%), add 1.6g of LiOH, then add 75g of purified water, 75g of tetrahydrofuran, and heat to 65°C under stirring to react, and control to the raw material by TLC After the reaction is complete, distill under reduced pressure (temperature is 30~35°C, vacuum degree is 250~350Pa) to remove the solvent, add 75ml of pure water to the remaining solid, adjust the pH to 2~3 with dilute hydrochloric acid, and divide it three times with 300ml of ethyl acetate. Extract, wash the organic phase with 50ml of saturated brine, and dry the organic phase with anhydrous sodium sulfate. Finally, concentrate (at a temperature of 30-35°C and a vacuum of 250-350Pa) the organic phase to obtain an off-white powder. The crude compound of formula (III) was recrystallized from methyl tert-butyl ether to obtain 14.5 g of white crystalline solid, HPLC: 99.5%, yield: 86.82%.
[0030] Step 2...
Embodiment 2
[0035] step 1:
[0036]
[0037] In a 250ml three-neck flask, add 17.5g of the compound of formula (II-2) (HPLC: 99.5%), add 2.3g of LiOH, then add 75g of purified water, 65g of methanol, and heat to 65°C under stirring to react, and control to the raw material by TLC After the reaction is complete, distill under reduced pressure (temperature is 30~35°C, vacuum degree is 250~350Pa) to remove the solvent, add 75ml of pure water to the remaining solid, adjust the pH to 2~3 with dilute hydrochloric acid, and divide it three times with 300ml of ethyl acetate. Extract, wash the organic phase with 50ml of saturated brine, and dry the organic phase with anhydrous sodium sulfate. Finally, concentrate (at a temperature of 30-35°C and a vacuum of 250-350Pa) the organic phase to obtain an off-white powder. The crude compound of formula (III) was recrystallized from methyl tert-butyl ether to obtain 14.9 g of white crystalline solid, HPLC: 99.4%, yield: 89.6%.
[0038] Step 2:
[00...
Embodiment 3
[0043] step 1:
[0044]
[0045] In a 250ml three-neck flask, add 20.0g of the compound of formula (II-3) (HPLC: 99.5%), add 1.7g of LiOH, then add 75g of purified water, 65g of 1,4-dioxane, and heat to 65°C under stirring Reaction, controlled by TLC until the reaction of the raw materials is complete, then distilled under reduced pressure (temperature 30~35°C, vacuum degree 250~350Pa) to remove the solvent, add 75ml of pure water to the remaining solid, adjust the pH to 2~3 with dilute hydrochloric acid, and then Extract three times with 300ml ethyl acetate, wash the organic phase with 50ml saturated brine, and dry the organic phase with anhydrous sodium sulfate. Finally, concentrate (at a temperature of 30-35°C and a vacuum of 250-350Pa) the organic phase to obtain an off-white powder. That is, the crude compound of formula III was recrystallized from methyl tert-butyl ether to obtain 15.0 g of white crystalline solid, HPLC: 99.2%, yield: 89.7%.
[0046] Step 2:
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com