Pitavastatin calcium tablet and preparation method thereof
A technology of pitavastatin calcium and tablet cores, which is applied in the field of medicine, can solve the problems of easily changing appearance, content uniformity, low content of pitavastatin calcium, and poor product stability, and achieve high dissolution rate, excellent drug properties, and stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A kind of pitavastatin calcium tablet, comprises tablet core and coating layer, each component and content of its tablet core are as follows (by 1000 tablets):
[0044] components Content per 1000 tablets (g) pitavastatin calcium 2 lactose 95 magnesium aluminum silicate 2.4 hypromellose 2 Low-substituted hydroxypropyl cellulose (internal addition) 9 Low-substituted hydroxypropyl cellulose (additional) 9 Magnesium stearate 0.6
[0045] The preparation steps of pitavastatin calcium tablets are as follows:
[0046] (1): Preparation of raw and auxiliary materials: take respectively raw material pitavastatin calcium, auxiliary materials lactose, magnesium aluminum silicate, low-substituted hypromellose, hypromellose and magnesium stearate and pass through a 60-mesh sieve after pulverization, spare;
[0047] (2): the preparation of hypromellose aqueous solution: the hypromellose of 0.2kg is slowly added to the purified...
Embodiment 2
[0063] A kind of pitavastatin calcium tablet, comprises tablet core and coating layer, each component and content of its tablet core are as follows (by 1000 tablets):
[0064] components Content per 1000 tablets (g) pitavastatin calcium 2 lactose 95 Hydroxyapatite 2.4 hypromellose 2 Low-substituted hydroxypropyl cellulose (internal addition) 9 Low-substituted hydroxypropyl cellulose (additional) 9 Magnesium stearate 0.6
[0065] The difference from Example 1 is that the protective agent aluminum magnesium silicate in Example 1 is replaced by hydroxyapatite, compared to the aluminum magnesium silicate used in Example 1 as the protective agent, the present embodiment uses Hydroxyapatite is a substance with a porous structure and has a large capacity for the main drug. After it is mixed with pitavastatin calcium, it can adsorb pitavastatin calcium in the internal pores to avoid or reduce its contact with the external environ...
Embodiment 3
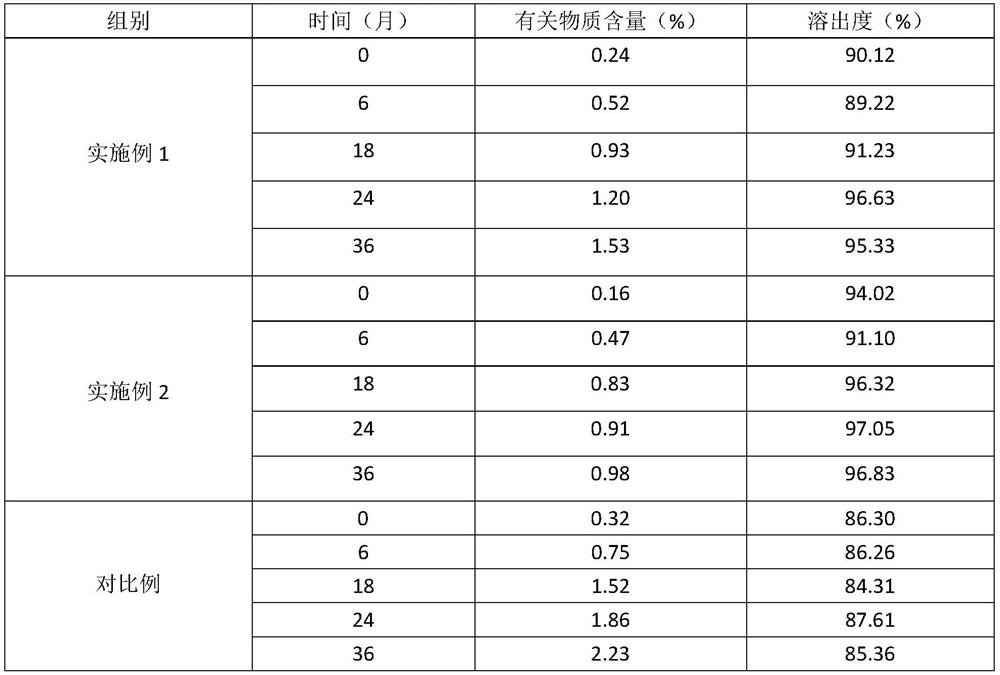
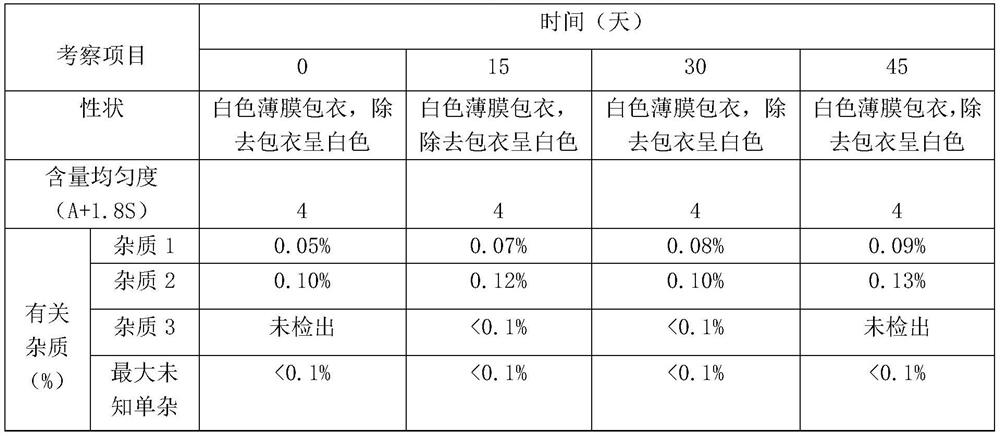
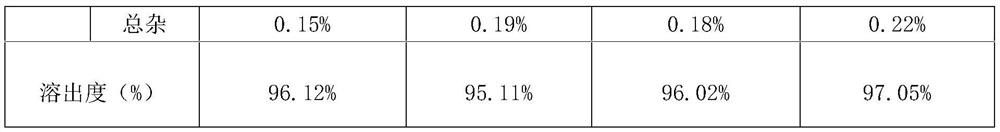
[0081] Pitavastatin calcium tablets were prepared according to the preparation method in Example 2 above, and the stability of the prepared pitavastatin calcium tablets was investigated in different conditions and ways. The experimental results are shown in the following table 2-5;
[0082] Wherein, the reference agent is: (trade name: Li Qingzhi, batch number 1050, manufacturer: Japan Xinghe Co., Ltd.).
[0083] Table 2 is embodiment stability investigation result
[0084]
[0085]
[0086] Table 3 is the accelerated experiment investigation result of embodiment (40 ± 2 ℃ of temperature, relative humidity 75% ± 5%)
[0087]
[0088] Table 4 is the investigation result of comparative example accelerated experiment (40 ± 2 ℃ of temperature, relative humidity 75% ± 5%)
[0089]
[0090] Table 5 is the long-term experimental investigation result of embodiment (30 ± 2 ℃ of temperature, relative humidity 60% ± 5%)
[0091]
[0092] As can be seen from the above exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com