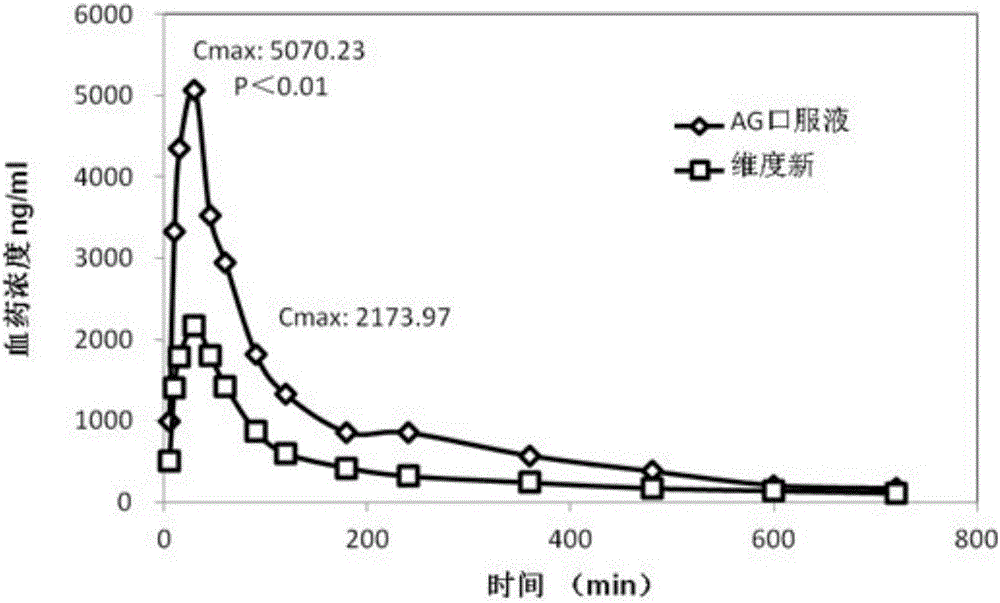
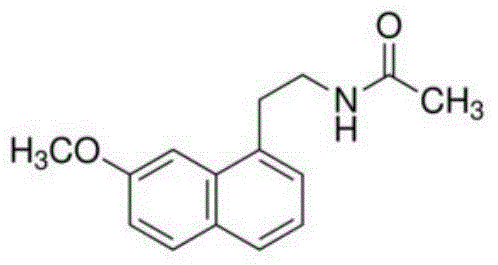
Agomelatine oral liquid preparation as well as preparation method and application thereof
A technology for agomelatine and liquid preparations, which is applied in the field of agomelatine preparations, which can solve the problems of not being able to reach the solubilization level of agomelatine, and achieve the reduction of individual absorption differences, rapid and uniform absorption, and stability good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] Add appropriate amount of water to hydroxypropyl-β-cyclodextrin, heat and stir at 70°C and 600rpm to dissolve, then add agomelatine, continue heating and stirring for 5 hours, add preservatives methyl paraben and propyl paraben Stir to dissolve the ester, cool to room temperature, and dilute to 500ml with ultrapure water, shake well to obtain a colorless, clear and transparent agomelatine oral solution, the pH of which is 7.1 after testing.
[0057] Under sterile conditions, the solution was filtered through a 0.22 μm filter membrane into a sterile container, subpackaged and stored at 4°C and 60°C, and the precipitation and purity content stability were investigated on the 10th day. The sample was stable at 4°C, no crystals of agomelatine precipitated, and the content of related substances passed the test after 10 days at 60°C.
[0058] The average degree of substitution of hydroxypropyl-β-cyclodextrin is 3.5.
[0059] The crystal form of agomelatine is cry...
Embodiment 2
[0061]
[0062] Add appropriate amount of water to hydroxypropyl-β-cyclodextrin, heat and stir at 55°C and 700rpm to dissolve, add agomelatine, continue heating and stirring for 2.5h, add preservatives methyl paraben and paraben Propyl ester, stirred until dissolved, cooled to room temperature, dilute to 500ml with ultra-pure water, shake well to obtain colorless, clear and transparent agomelatine oral solution, pH is 7.1 after testing.
[0063] Under sterile conditions, the solution was filtered through a 0.22 μm filter membrane into a sterile container, subpackaged and stored at 4°C and 60°C, and the precipitation and purity content stability were investigated on the 10th day. The sample was stable at 4°C, no crystals of agomelatine precipitated, and the content of related substances passed the test after 10 days at 60°C.
[0064] The average degree of substitution of hydroxypropyl-β-cyclodextrin is 6.0.
Embodiment 3
[0067]
[0068] Add appropriate amount of water to hydroxypropyl-β-cyclodextrin, heat and stir at 65°C and 800rpm to dissolve, then add agomelatine powder, continue heating and stirring for 1.5h, add preservative sorbic acid, and add sodium hydroxide to adjust pH , stir until dissolved, after cooling to room temperature, dilute to 500ml with ultrapure water, shake well to obtain a colorless, clear and transparent agomelatine oral solution, the pH of which is 4.5 after testing.
[0069] Under sterile conditions, the solution was filtered through a 0.22 μm filter membrane into a sterile container, subpackaged and stored at 4°C and 60°C, and the precipitation and purity content stability were investigated on the 10th day. The sample was stable at 4°C, no crystals of agomelatine precipitated, and the content of related substances passed the test after 10 days at 60°C.
[0070] The average degree of substitution of hydroxypropyl-β-cyclodextrin was 5.0.
[0071] Agomelatine is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com