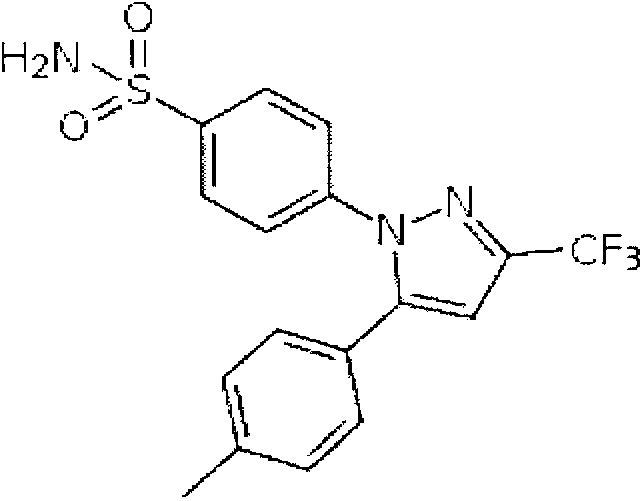
Celecoxib new formulation and preparation method thereof
A technology of celecoxib and preparation, which is applied to the new dosage form of celecoxib and its preparation field, can solve the problems of easy agglomeration, low solubility of celecoxib, and cannot be directly compressed, and achieves increased drug efficacy , good release reproducibility, small difference in loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 (sustained-release pellets)
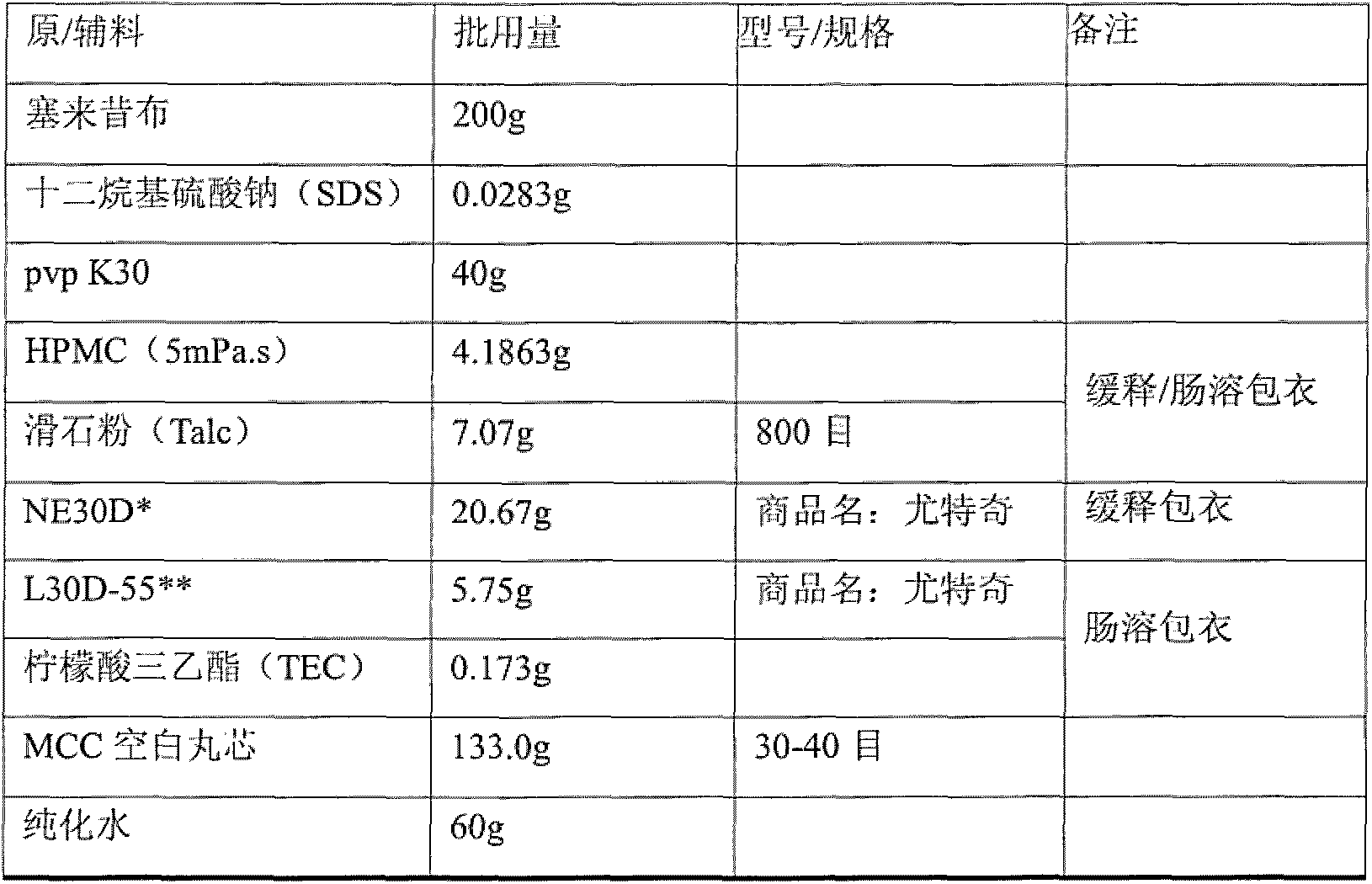
[0039] The prescription is as follows
[0040]
[0041]
[0042] A. Prepare the solution according to the prescription
[0043] A-1 preparation of drug-loading solution
[0044]Weigh the celecoxib raw material and sodium lauryl sulfate in a beaker according to the prescription, then add 95% ethanol in the prescribed amount, and stir until the celecoxib raw material is completely dissolved. Remove the beaker from the water bath, let it stand, and cool to room temperature. Finally, slowly add pvpK30 according to the prescription, stir and let stand until pvpK30 is completely dissolved.
[0045] A-2 Preparation of isolation coating solution
[0046] Weigh 50% hydroxypropyl methylcellulose HPMC (5mPa.s) according to the prescription, take an appropriate amount of purified water, slowly add HPMC to the water, stir while adding, and let it stand until the HPMC is completely swollen.
[0047] A-3 preparation of sustained-re...
Embodiment 2
[0077] Embodiment 2 (enteric-coated pellets)
[0078] Prescription sees embodiment 1 form, and processing step is with embodiment 1, and difference is:
[0079] A-3 preparation of enteric coating solution
[0080] Swell the remaining HPMC (5mPa.s) in an appropriate amount of purified water and set aside;
[0081] Weigh triethyl citrate (TEC) according to the prescription, add an appropriate amount of purified water, stir, then add the prescribed amount of talc powder (Talc, 800 mesh), stir, and undergo high-speed shear (10000rpm) for 10 minutes to obtain A;
[0082] Weigh L30D-55 according to the prescription, slowly add to A, stir while adding, and get B;
[0083] Mix the previously prepared HPMC solution into B, stir and mix well, and add the remaining purified water to obtain C;
[0084] Stir slowly with a paddle agitator C, pass through a 40-mesh sieve just before coating, keep stirring for no less than 40 minutes before coating, and keep stirring the coating solution d...
Embodiment 3
[0089] Embodiment 3 (sustained-release pellets+enteric-coated pellets)
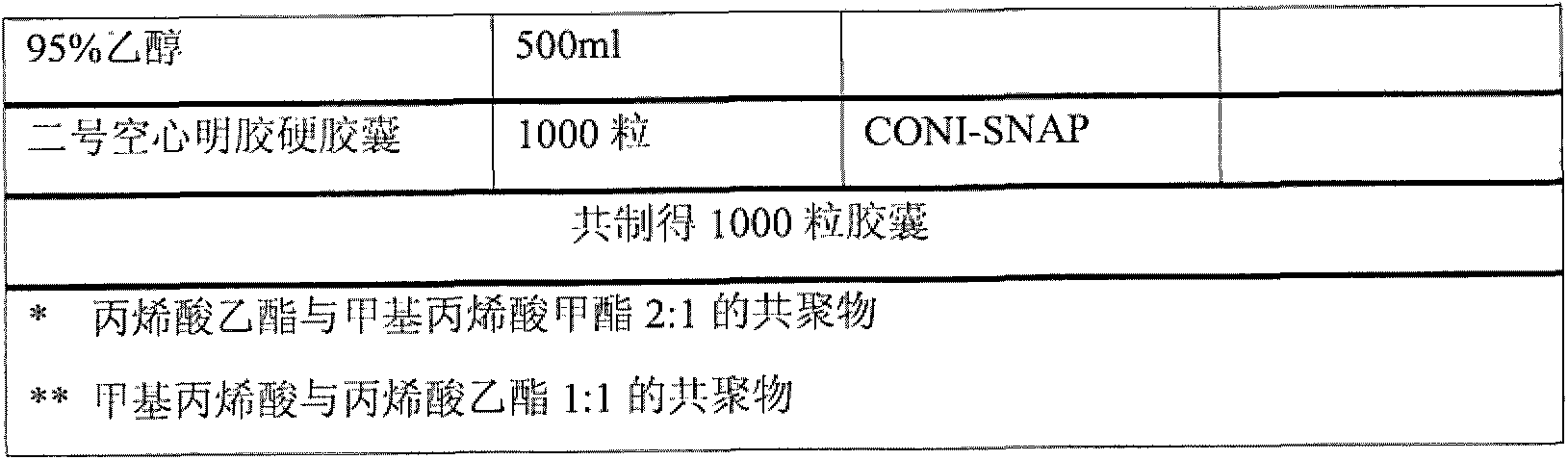
[0090] The sustained-release coated pellets prepared in Examples 1 and 2 were uniformly mixed with the enteric coated pellets at a ratio of 3:1, and packed into No. 2 capsules (200 mg specification).
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com