Medicine composition containing cinacalcet hydrochloride and production method of medicine composition containing cinacalcet hydrochloride
A technique for cinacalcet hydrochloride and its composition, which is applied in the field of pharmaceutical composition containing cinacalcet hydrochloride and its preparation, and can solve problems such as difficult popularization, high requirements for equipment and operation, and complicated preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Cinacalcet hydrochloride 25mg specification
[0017] According to the proportioning of table 1 and following process, Xi'an Caleb hydrochloride tablet is prepared, and dissolution rate is measured, and compared with commercially available cinacalcet hydrochloride tablet, data are listed in the following table:
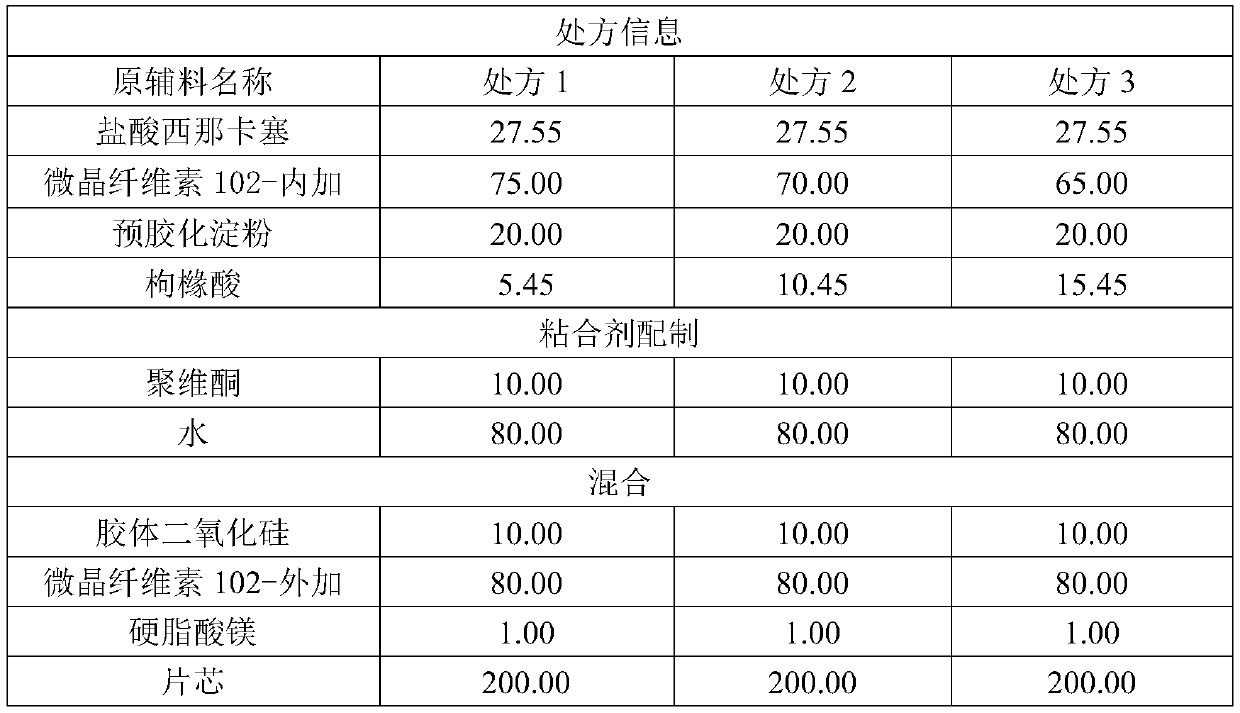
[0018] Table 1
[0019]
[0020] Tablet core preparation: Weigh the prescribed amount of cinacalcet hydrochloride, add microcrystalline cellulose 102, pregelatinized starch, and disintegrant citric acid to pass through a No. 3 sieve, mix well and add the viscose prepared by povidone K30. Mixture soft material, dried in fluidized bed or oven, granulated by granulator, added prescription amount of microcrystalline cellulose, colloidal silicon dioxide and mixed evenly, then added magnesium stearate and mixed for 3-5min, after tableting That is the tablet core.
[0021] Coating: Prepare a coating solution with a concentration of 8-16%, put the tablet core into ...
Embodiment 2
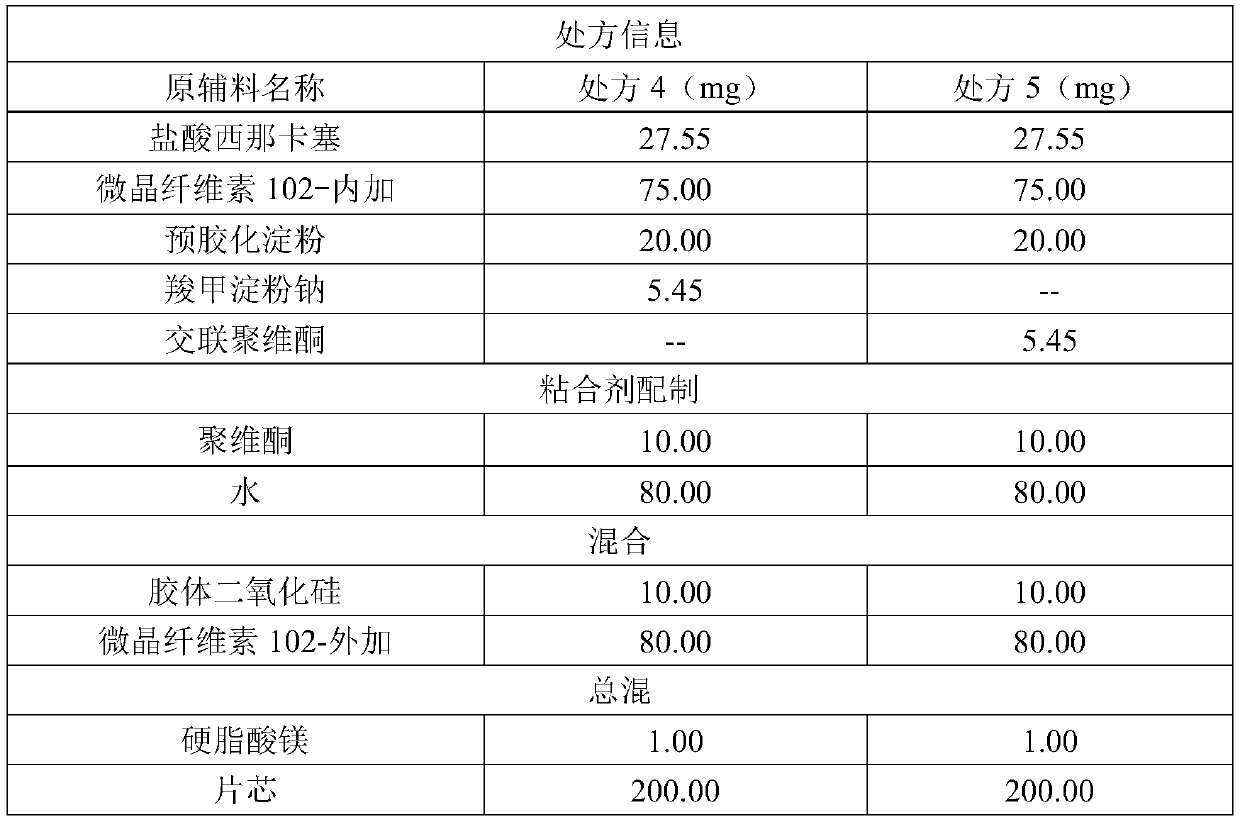
[0023] Cinacalcet hydrochloride 25mg specification
[0024] The preparation method of this 25mg cinacalcet tablet is the same as Example 1, except that the disintegrant citric acid in the tablet core is replaced with sodium carboxymethyl starch or crospovidone. The specific prescription composition is shown in Table 2 below.
[0025] Table 2
[0026]
Embodiment 3
[0028] Cinacalcet hydrochloride 25mg specification
[0029] The preparation method of this 25mg cinacalcet tablet is the same as embodiment 1, and difference is that the ratio of microcrystalline cellulose 102 in the tablet core is added inside and outside is different, and concrete prescription composition is shown in Table 3.
[0030] table 3
[0031]
[0032]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com