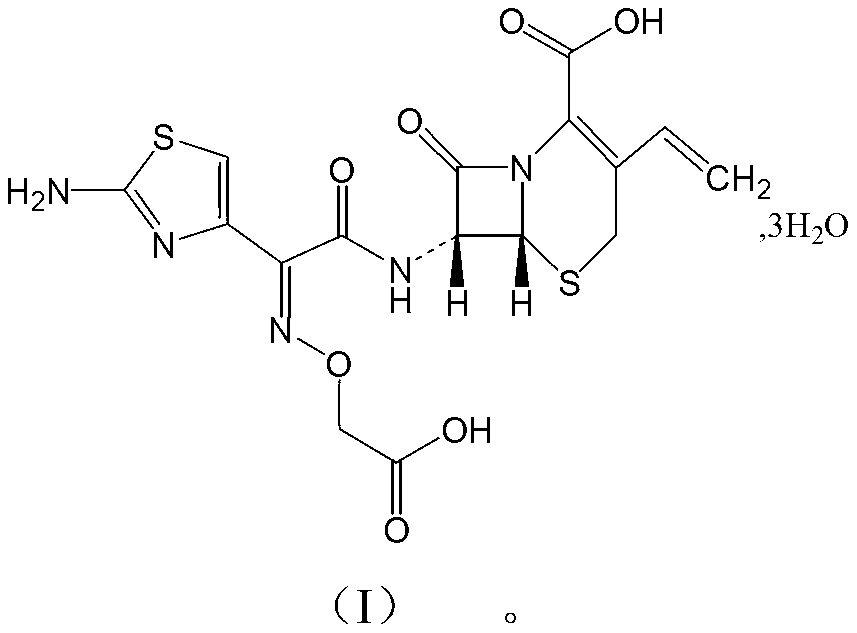
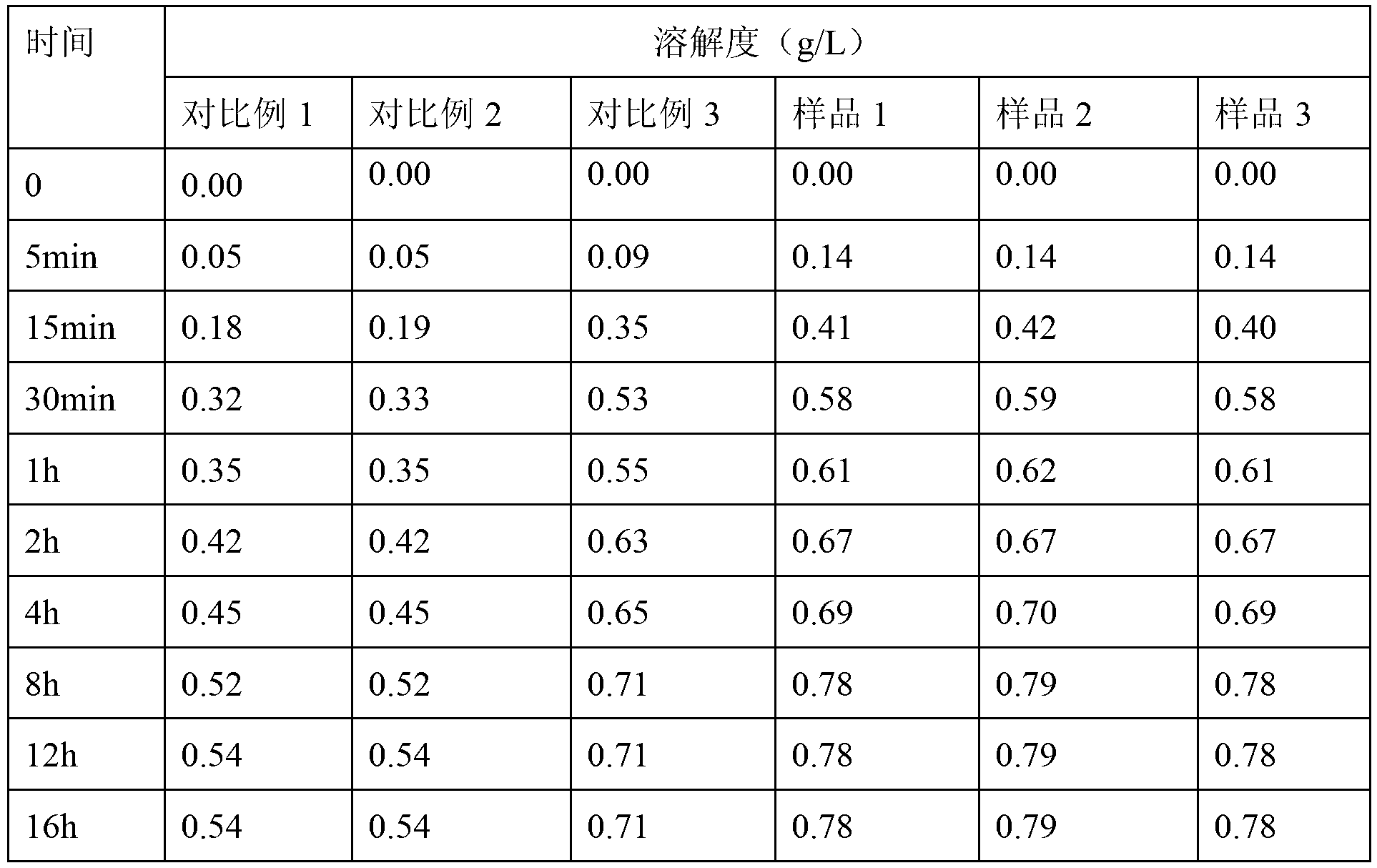
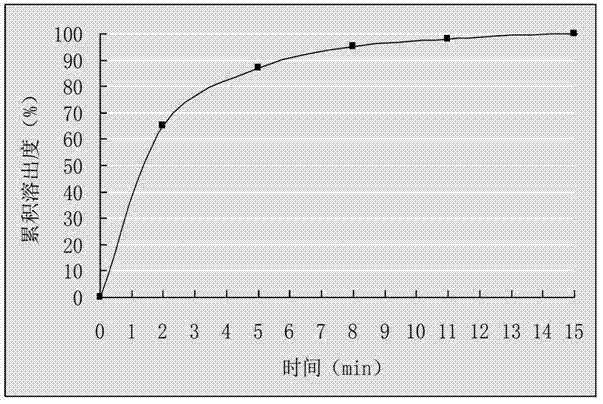
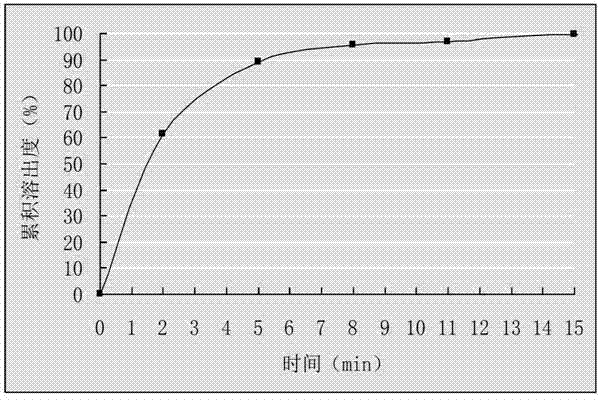
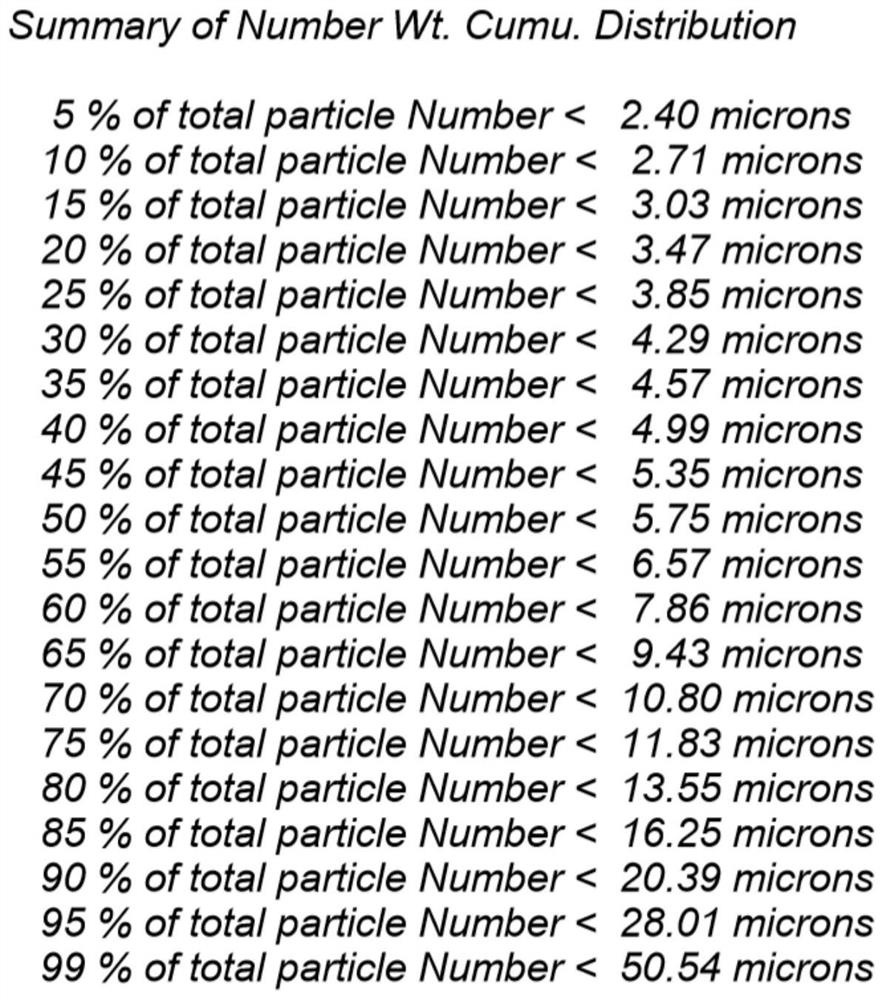
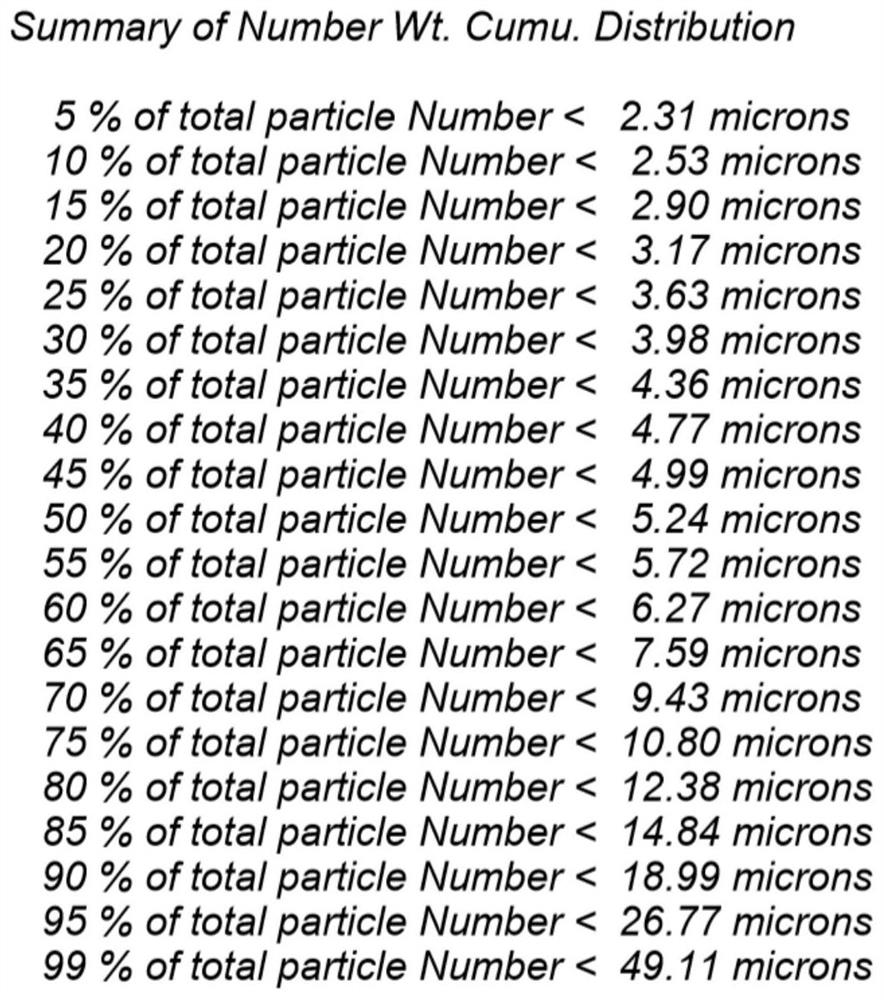
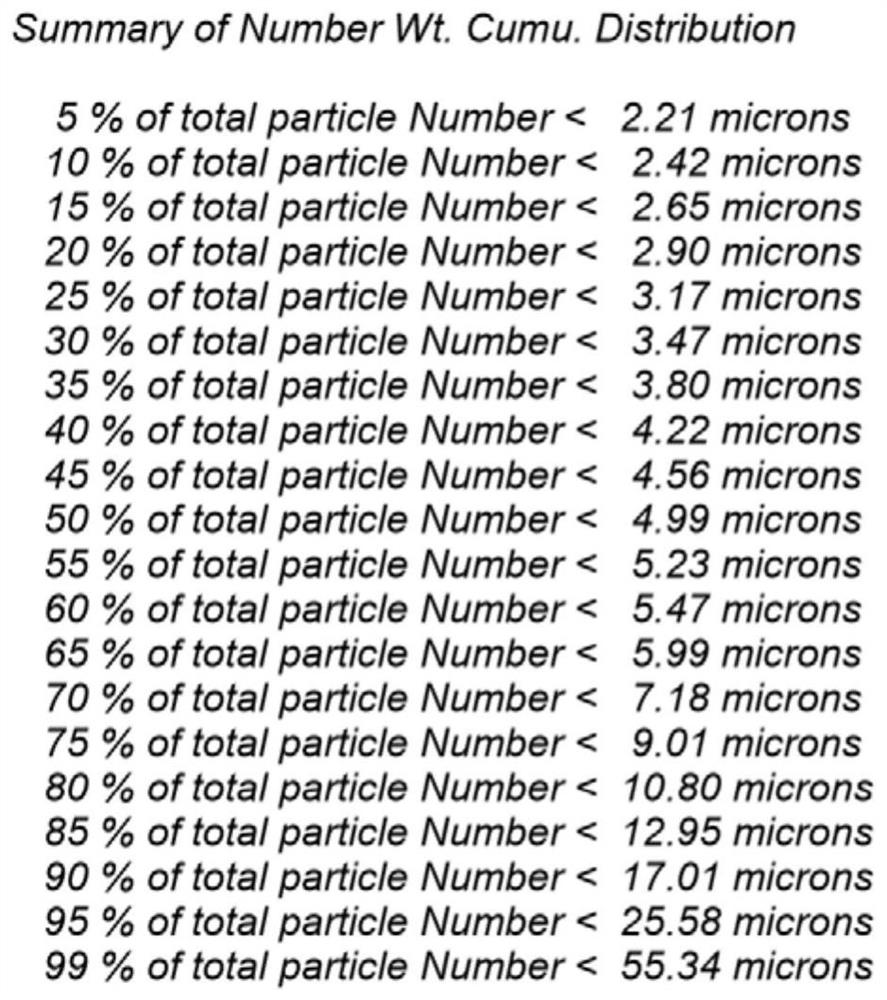
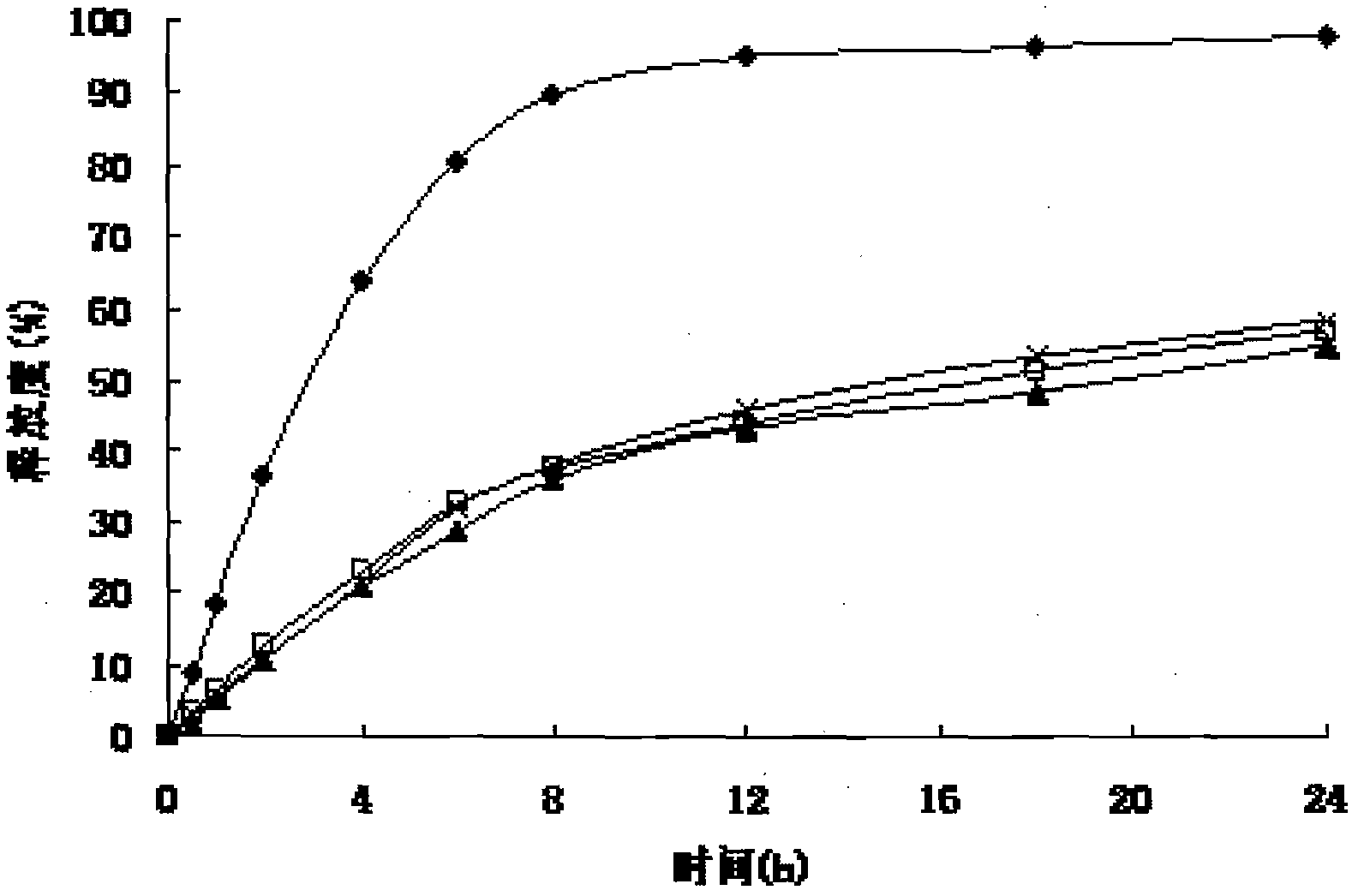
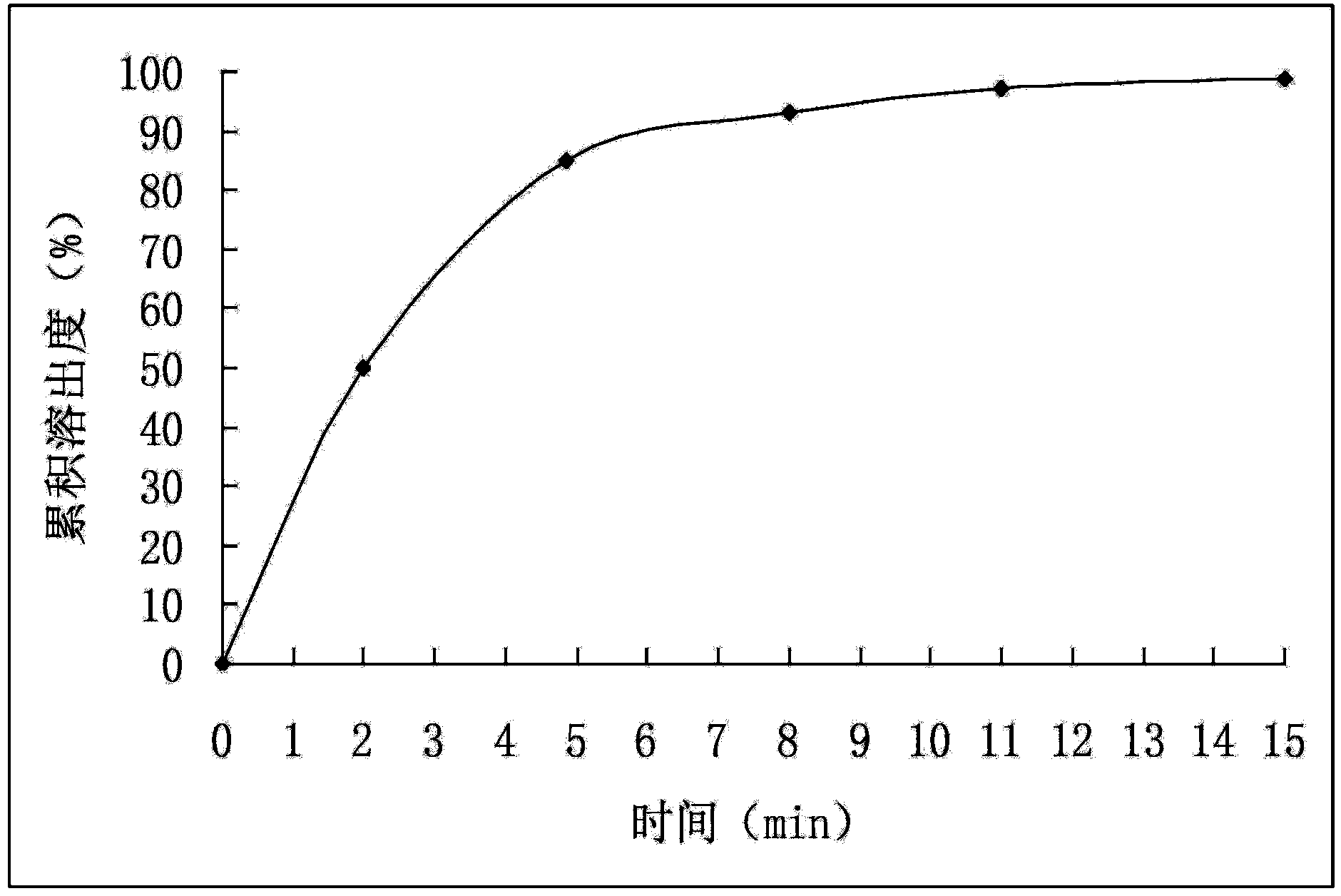
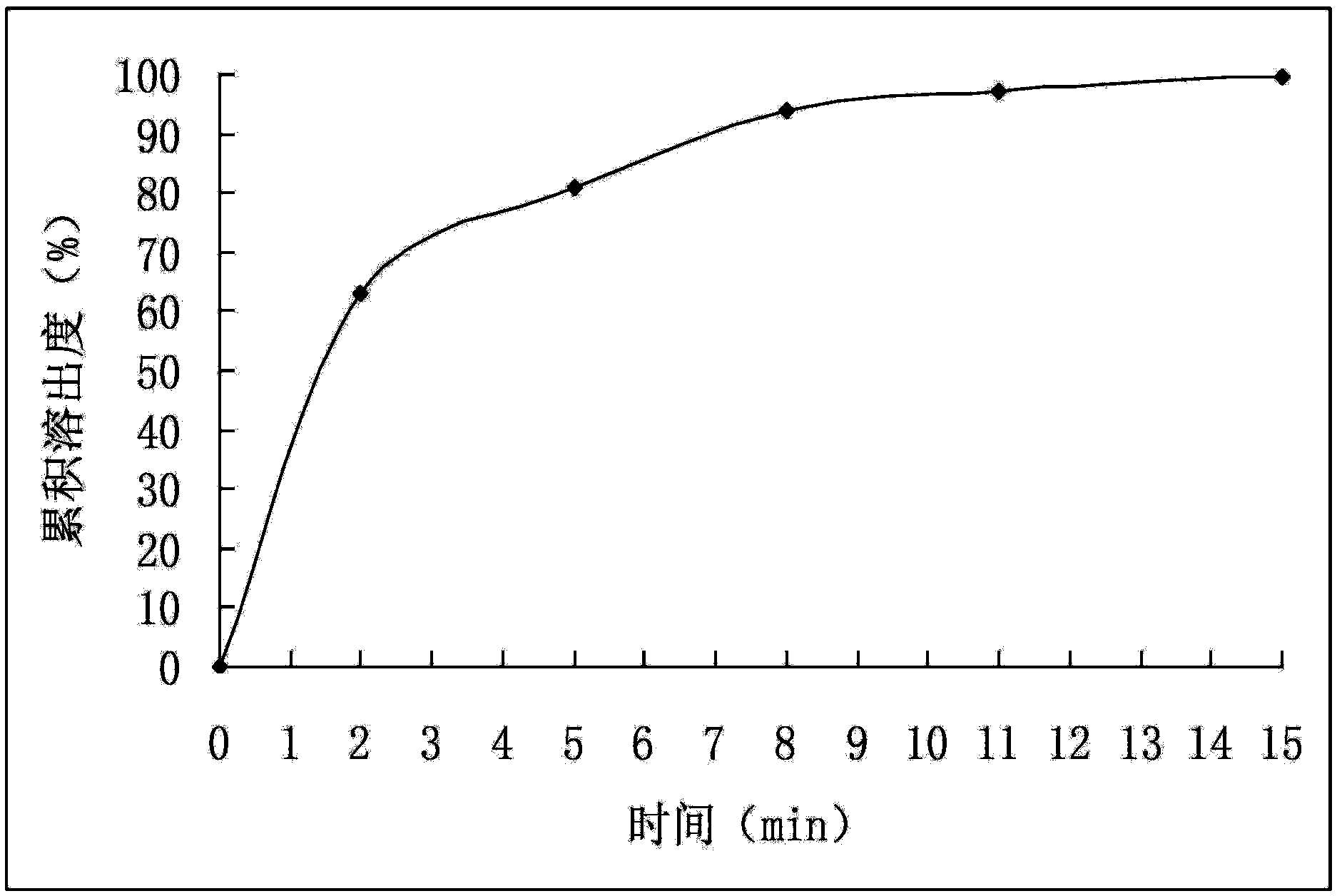
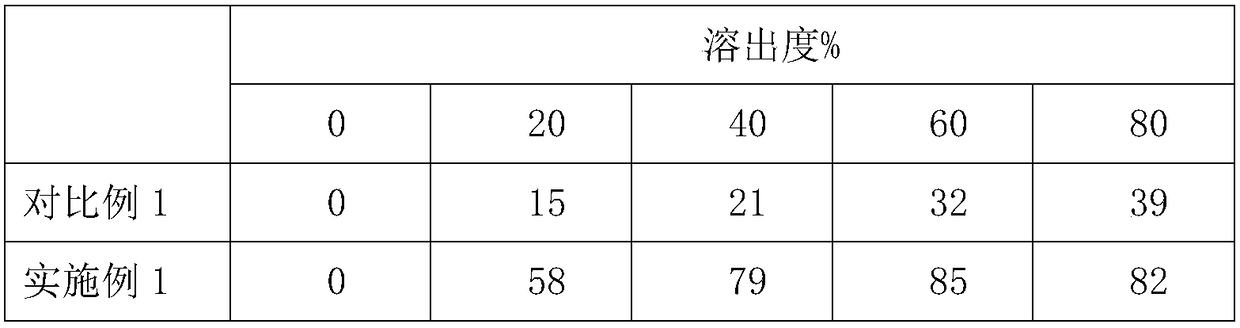
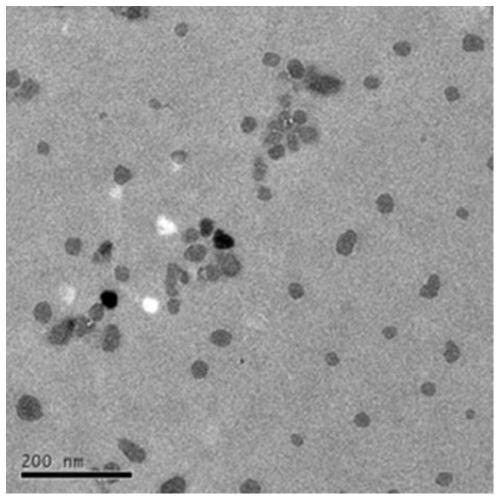
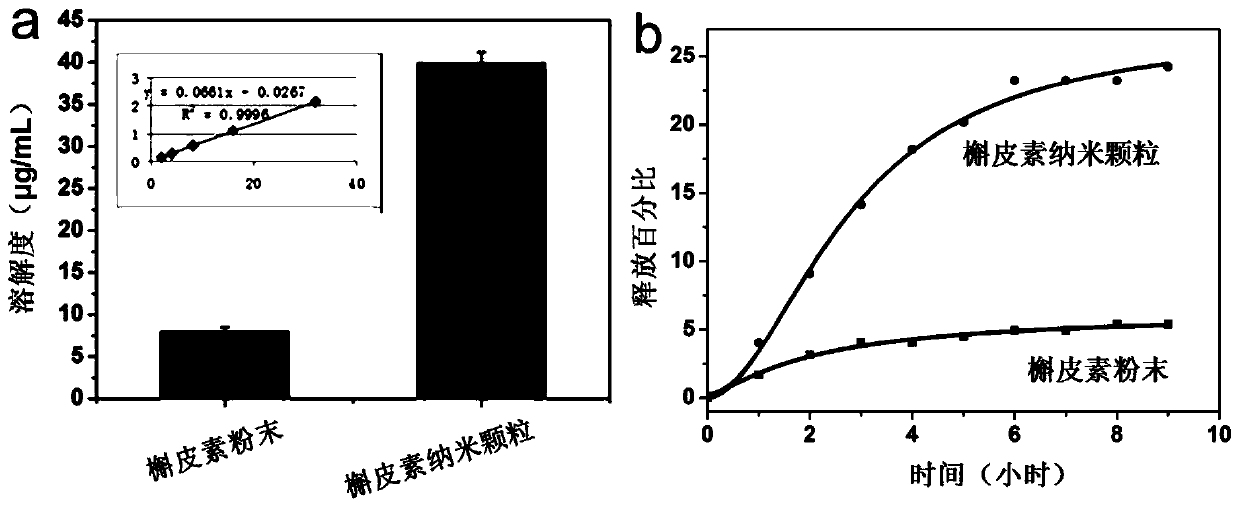
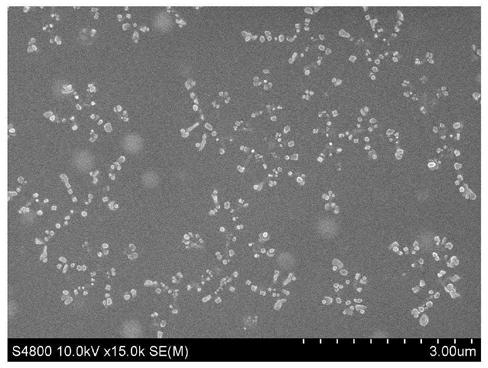
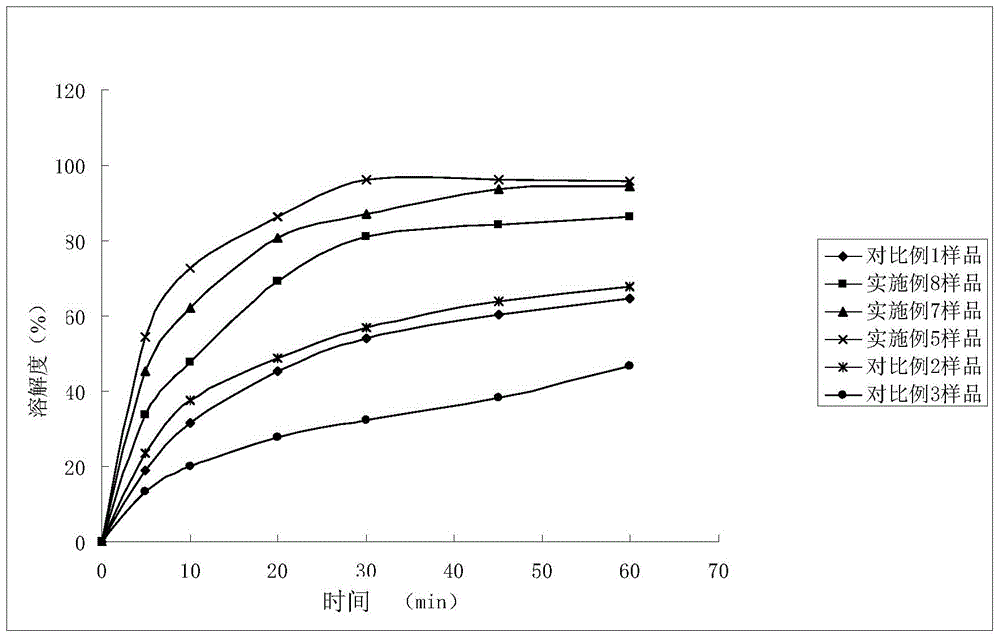
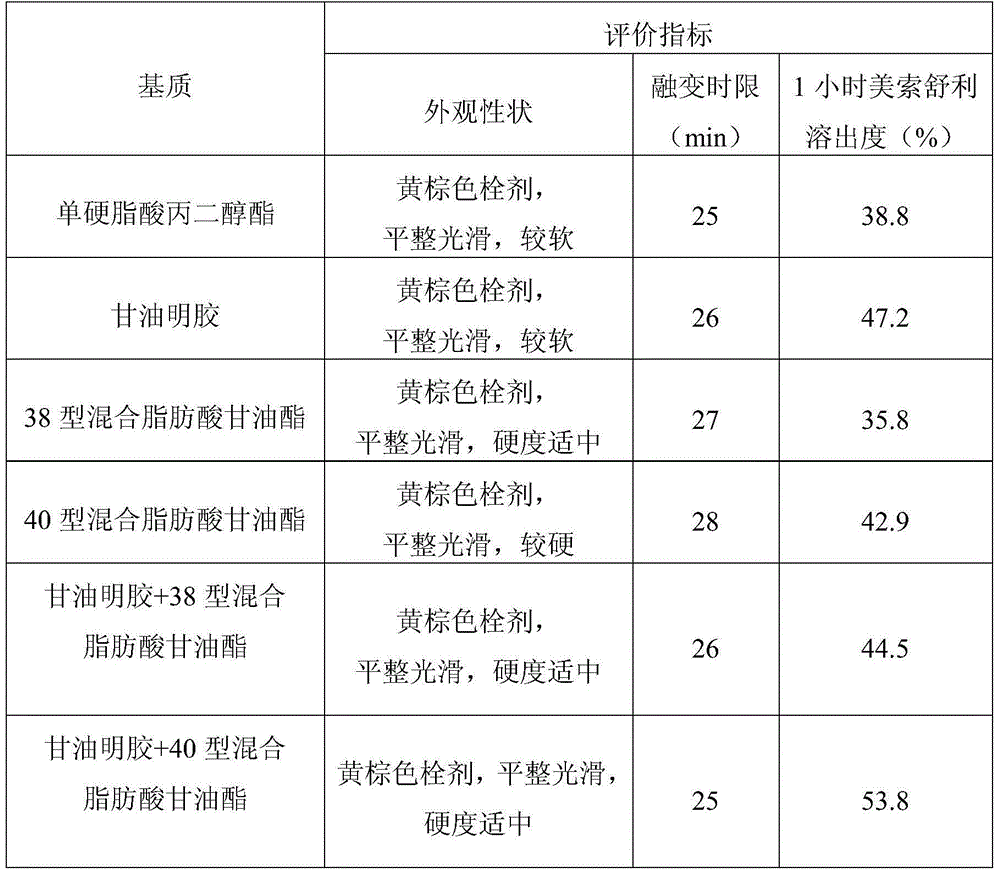
Patents
Literature
33results about How to "Improve drug dissolution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
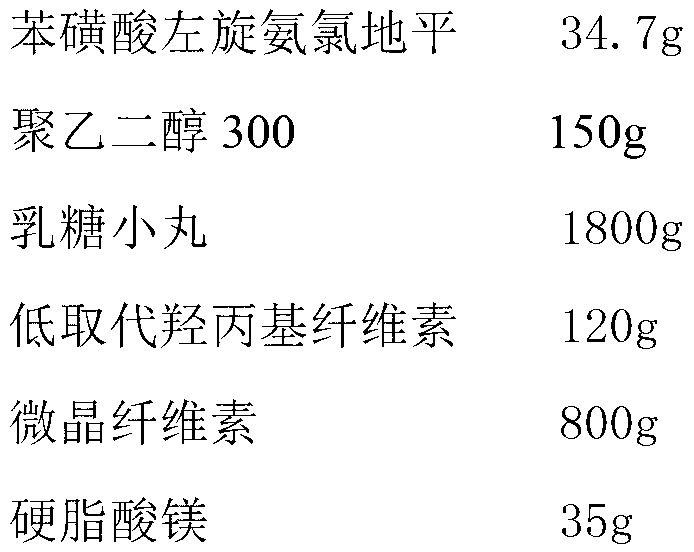
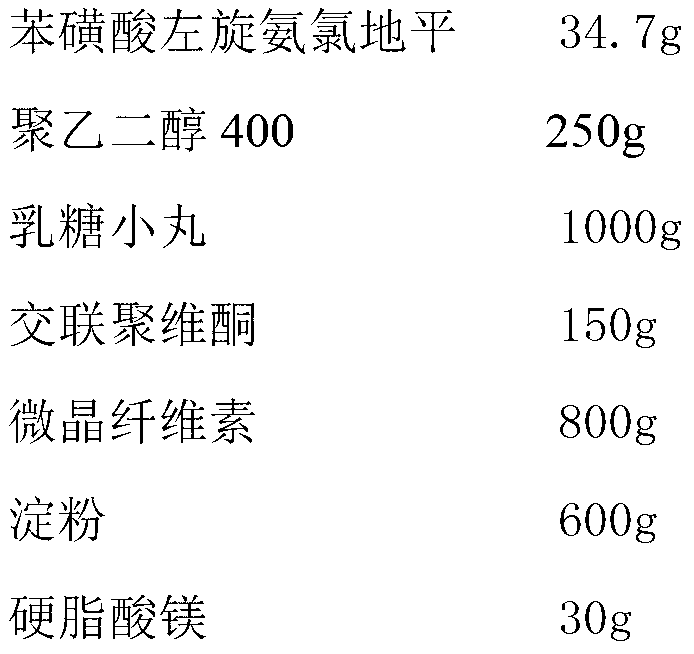
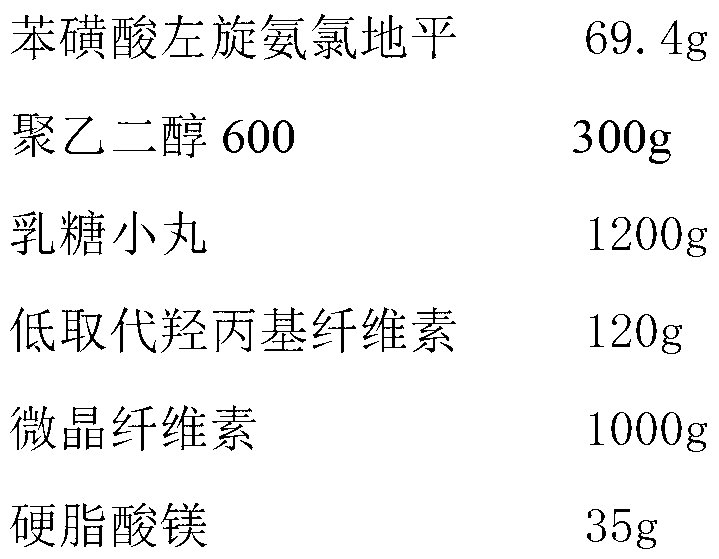
Levamlodipine besylate tablet and preparation method thereof
ActiveCN103127018AHigh specific surface areaImprove drug dissolution and bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsChemistryPolyethylene glycol
The invention discloses a levamlodipine besylate tablet. The tablet is prepared by mixing a medicine carrying pellet containing levamlodipine besylate and pharmaceutically-accepted accessories and then carrying out direct compression, wherein the medicine carrying pellet is prepared by using the following method comprising the following steps of: weighting levamlodipine besylate and polyethylene glycol according to the weight ratio of 1: (4-8), then, dissolving levamlodipine besylate and polyethylene glycol into an organic solvent; and spraying the formed solution on a lactose pellet in a fluidized bed, coating and drying to obtain the medicine carrying pellet. The levamlodipine besylate raw material is coated on the outer layer of the lactose pellet and medicine is uniformly distributed on the surface of the pellet, so that the specific surface area of the medicine is greatly increased; and the raw material can be rapidly released, and thus, the medicine dissolution rate and the bioavailability of the levamlodipine besylate tablet are increased.
Owner:ZHEJIANG ANGLIKANG PHARMA
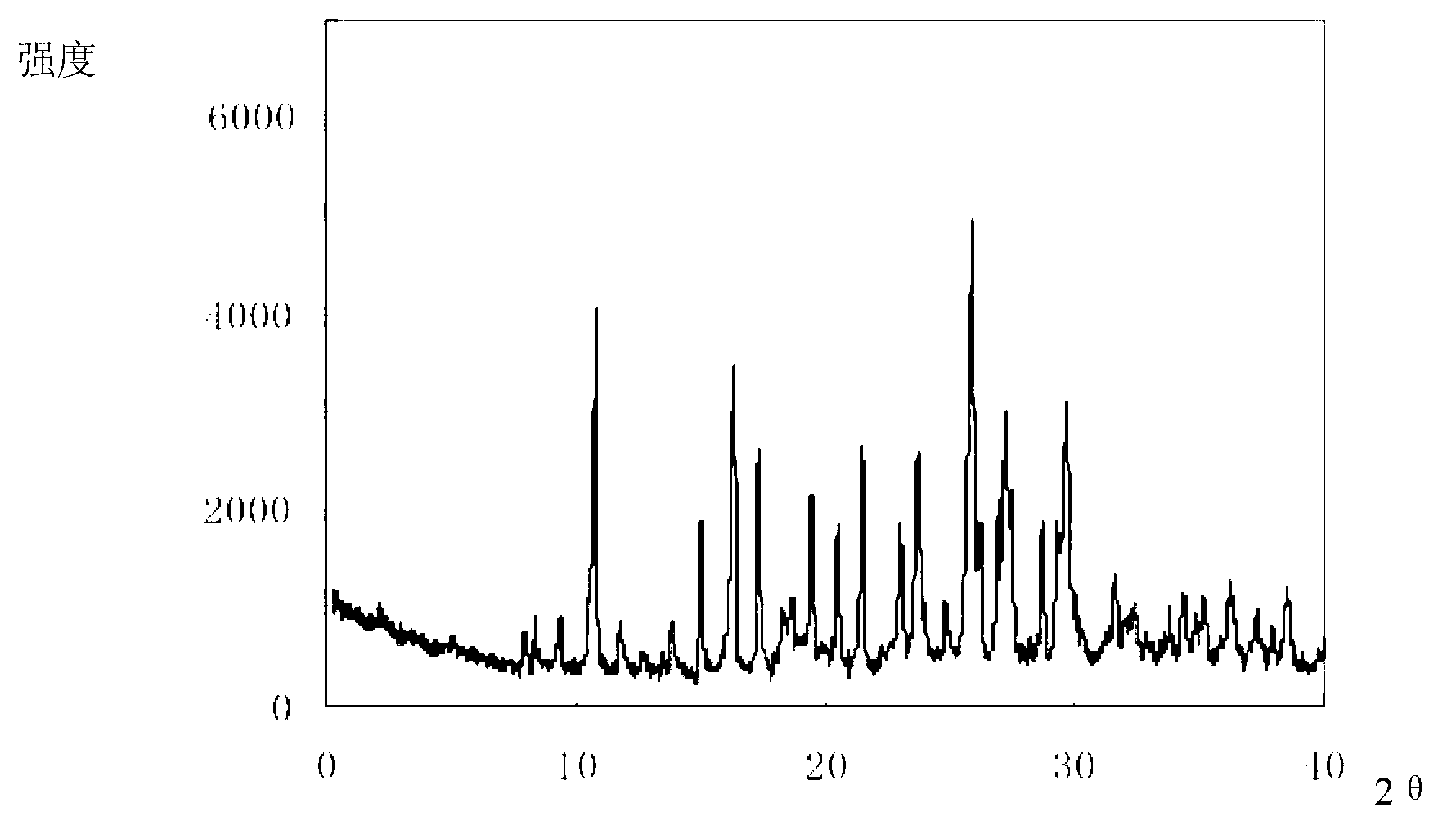
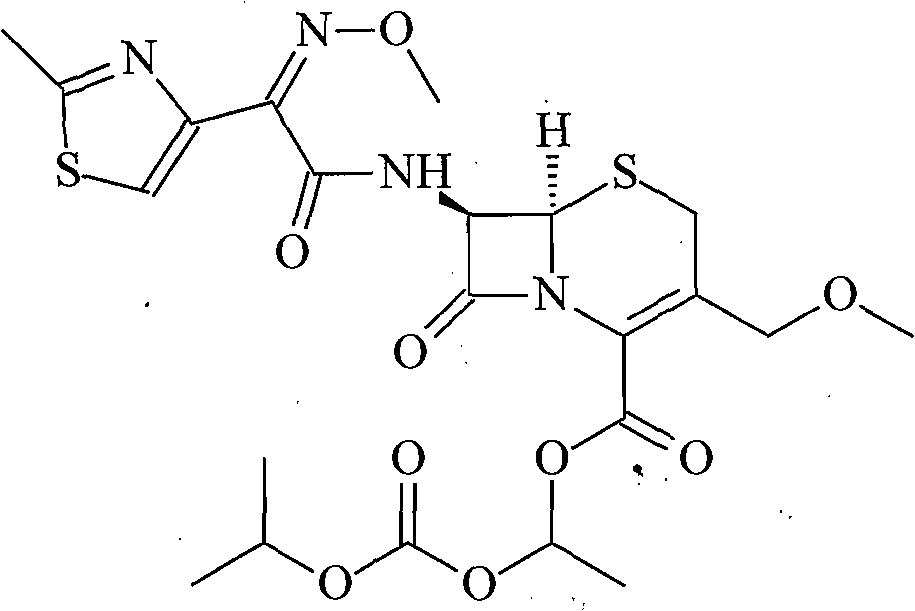
Cefixime compound and pharmaceutical composition thereof
InactiveCN103193798AUniform particle size distributionWell mixedAntibacterial agentsOrganic active ingredientsDrug compoundPharmaceutical Adjuvants
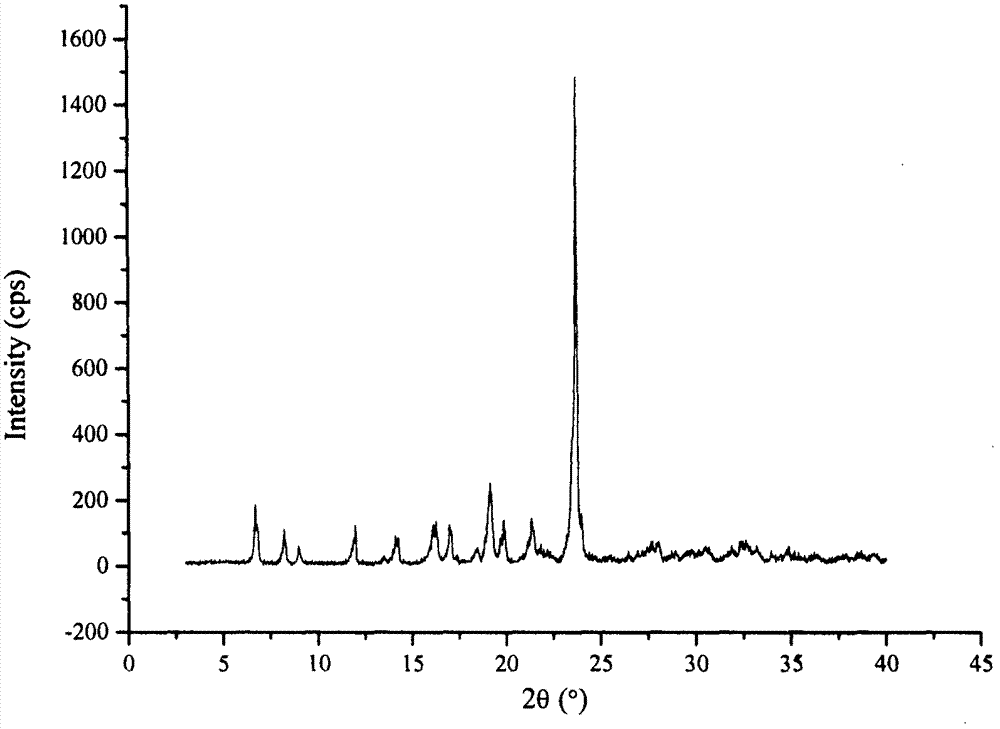
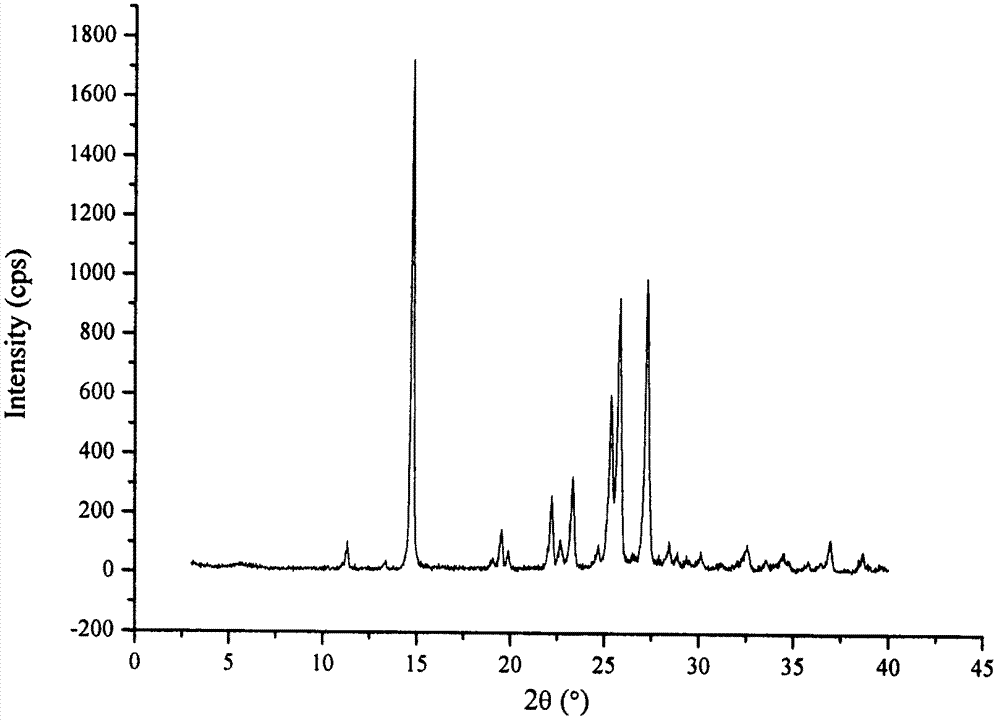
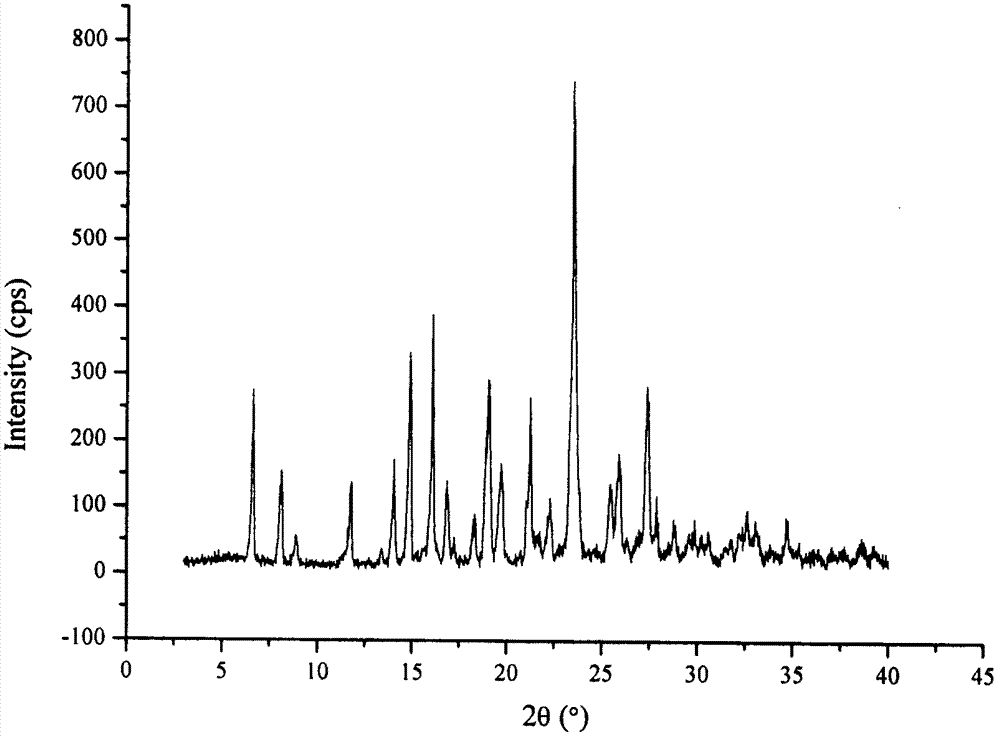
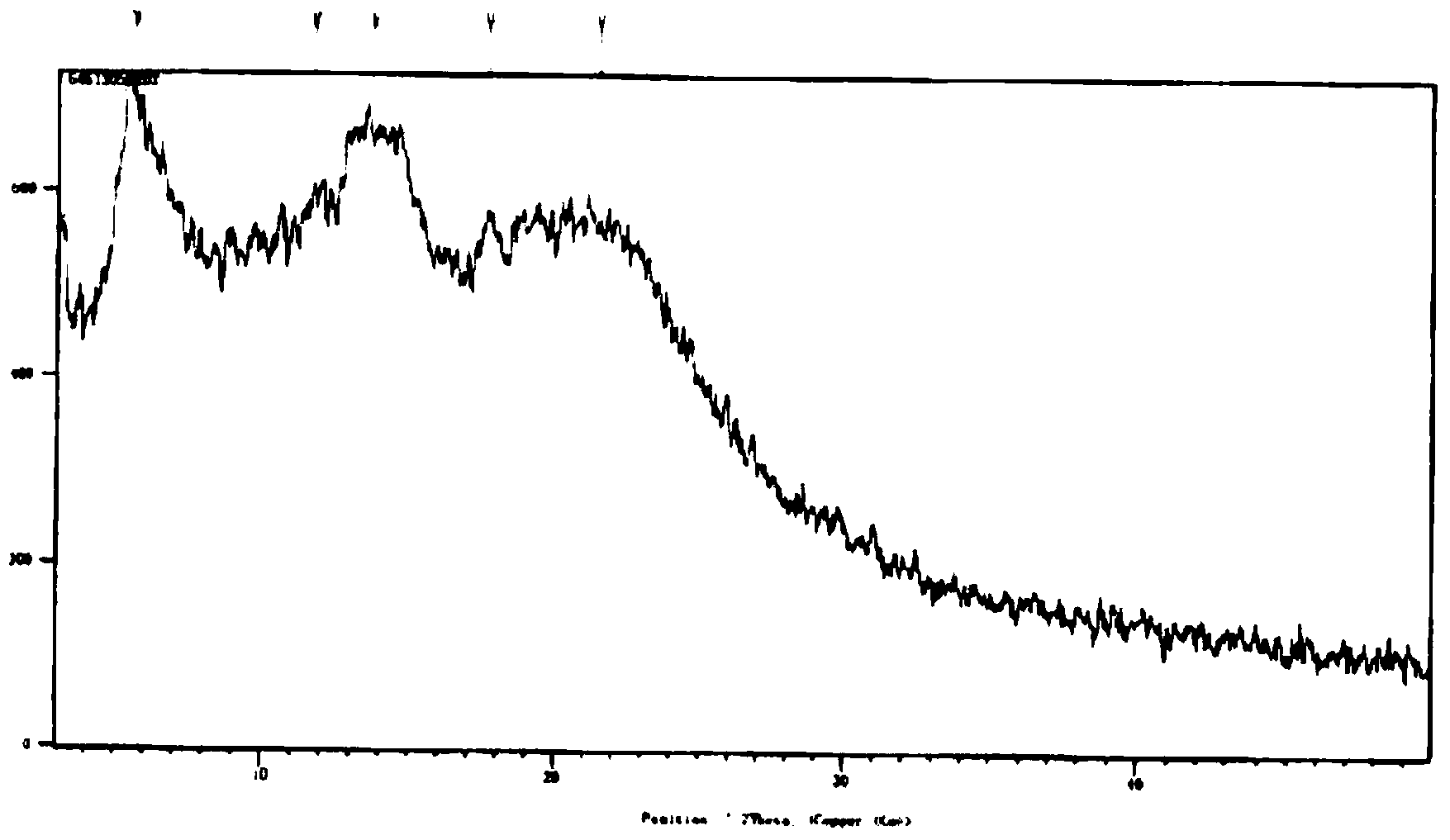
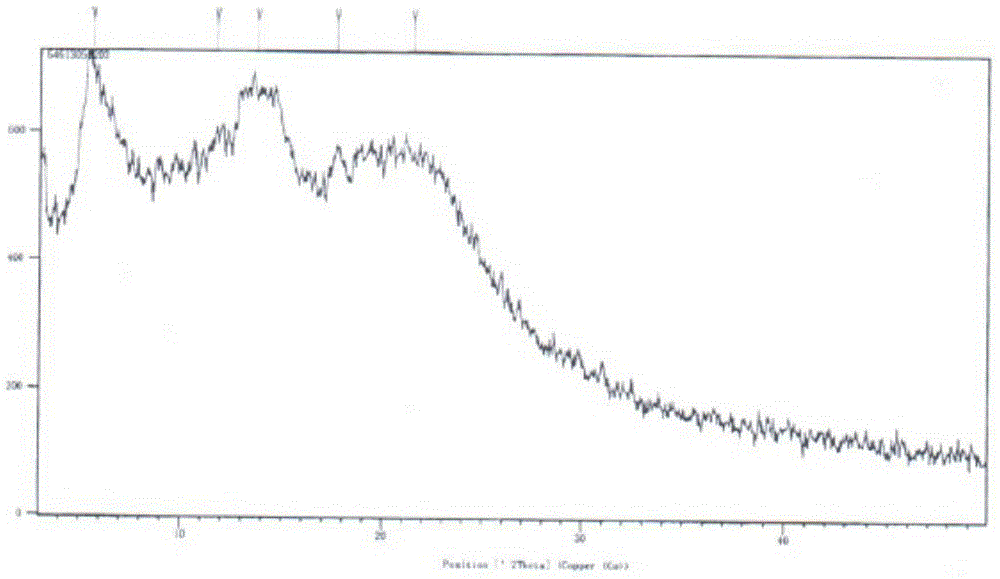
The invention relates to a drug compound, and particularly relates to a cefixime compound. An X-ray powder diffraction pattern of the cefixime compound measured through a Cu-K alpha ray is shown in a figure I. The invention also relates to a pharmaceutical composition containing the cefixime compound. The pharmaceutical composition comprises the cefixime compound and a pharmaceutical adjuvant; the pharmaceutical composition is an oral preparation including an oral normal release preparation and a controlled release preparation; and the oral normal release preparation is selected from a tablet, an enteric-coated tablet, a capsule, a dispersible tablet, dry suspension, a chewable tablet or granules. The cefixime compound disclosed by the invention is high in purity, high in bioavailability and suitable for clinical application.
Owner:四川省惠达药业有限公司
Cefuroxime axetil capsule and preparation method thereof
InactiveCN103040788AHigh specific surface areaImprove drug dissolutionAntibacterial agentsOrganic active ingredientsDrugInjectable Suspension
The invention discloses a cefuroxime axetil capsule. The cefuroxime axetil capsule is prepared by filling the uniformly mixed cefuroxime axetil-containing medicine-carrying pills and talcum powder into a capsule shell; and the medicine-carrying pills are prepared according to the following steps of: weighing the cefuroxime axetil and a disintegrating agent to be dissolved or dispersed in acetone, spraying the formed solution or injectable suspension to lactose pills in a fluidized bed, and coating and drying to obtain the medicine-carrying pills. The cefuroxime axetil raw material is coated on the outer layers of the lactose pills and is uniformly distributed on the surfaces of the pills, the specific surface area of the medicine is greatly increased, the raw material can be quickly released, and thus, the dissolubility and bioavailability of the medicine are improved.
Owner:NANJING ZHENGKUAN MEDICAL TECH
A Qi and blood tonifying functional beverage
InactiveCN104997087AImprove immunityImprove drug dissolutionMetabolism disorderDigestive systemBiotechnologyFermentation
The present invention discloses a Qi and blood tonifying functional beverage and a preparation method thereof. The functional beverage consists of the following components in parts by weight: 12-18 parts of donkey-hide gelatin, 8-10 parts of rhizoma polygonati, 12-18 parts of Chinese yam,14-16 parts of Chinese wolfberry fruits, 8-12 parts of mulberries, 50-70 parts of honey, 50-70 parts of white granulated sugar and 5,000-6,000 parts of water. Through fermenting ingredients of a reasonable medicinal formula and breaking wall by fermentation, the drug effect is enhanced, and through a combined fermentation of zygosaccharomyces bailii and bacillus xylinus, a variety of vitamins, amino acids, trace elements and ingredients beneficial to human body contained in raw materials are dissolved out without adding any hormones or chemical compositions, thus the Qi and blood tonifying effect is achieved, and effects in physical health, strengthening body, and enhancing body immunity are also achieved after a user drinks the functional beverage. The functional beverage is suitable for both males and females, and the promotion of the functional beverage benefits the nation and the people.
Owner:杜圣艳
Positively charged drug nanocrystal preparation and preparation method thereof
InactiveCN109331184AExtended stayImprove stabilityAerosol deliveryOintment deliveryDrugs preparationsNanocrystal
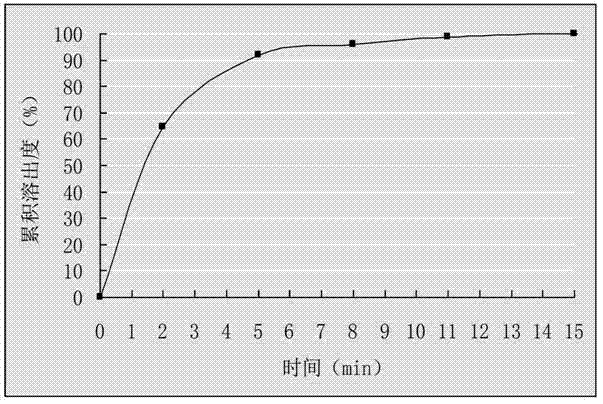
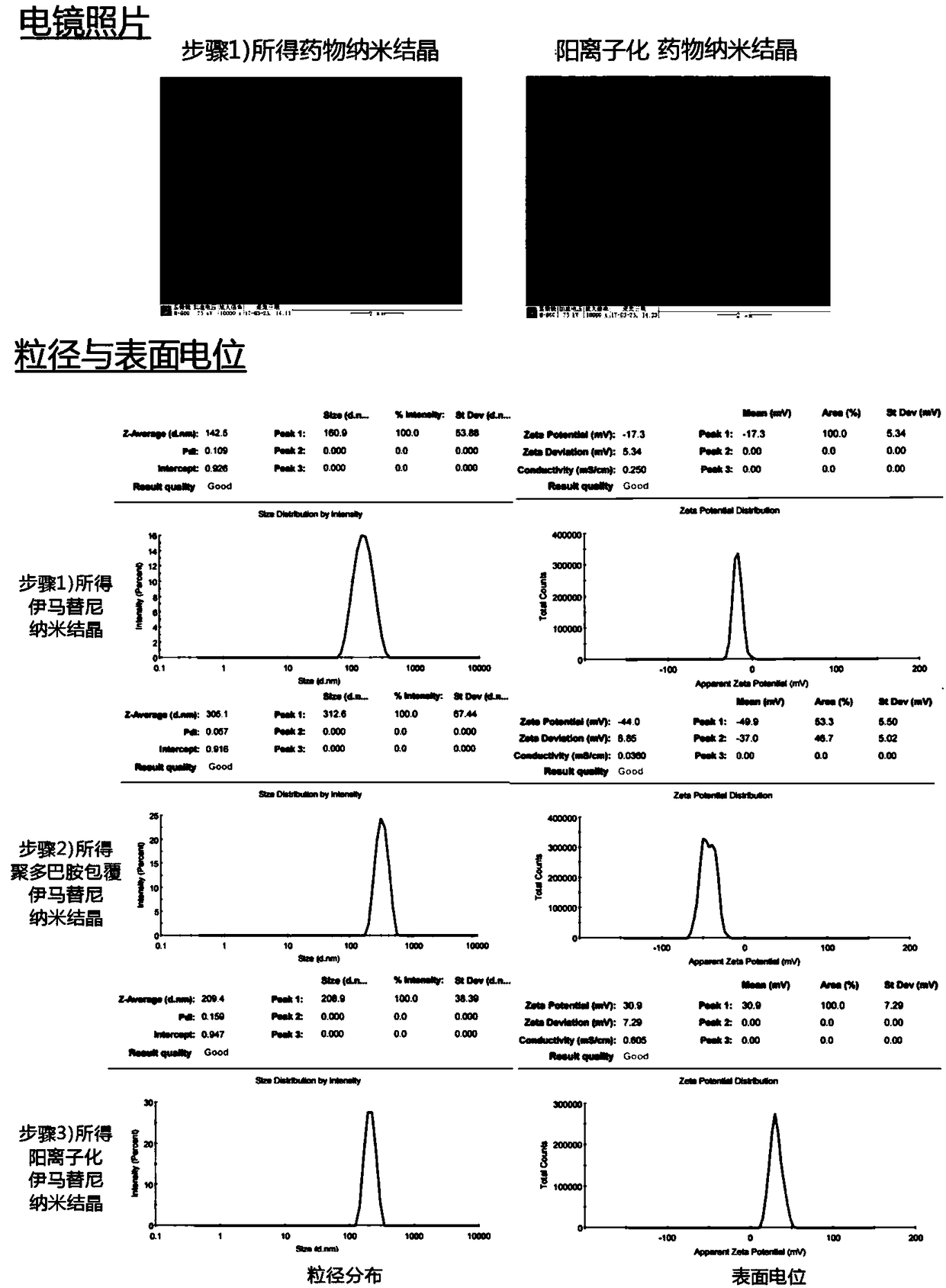
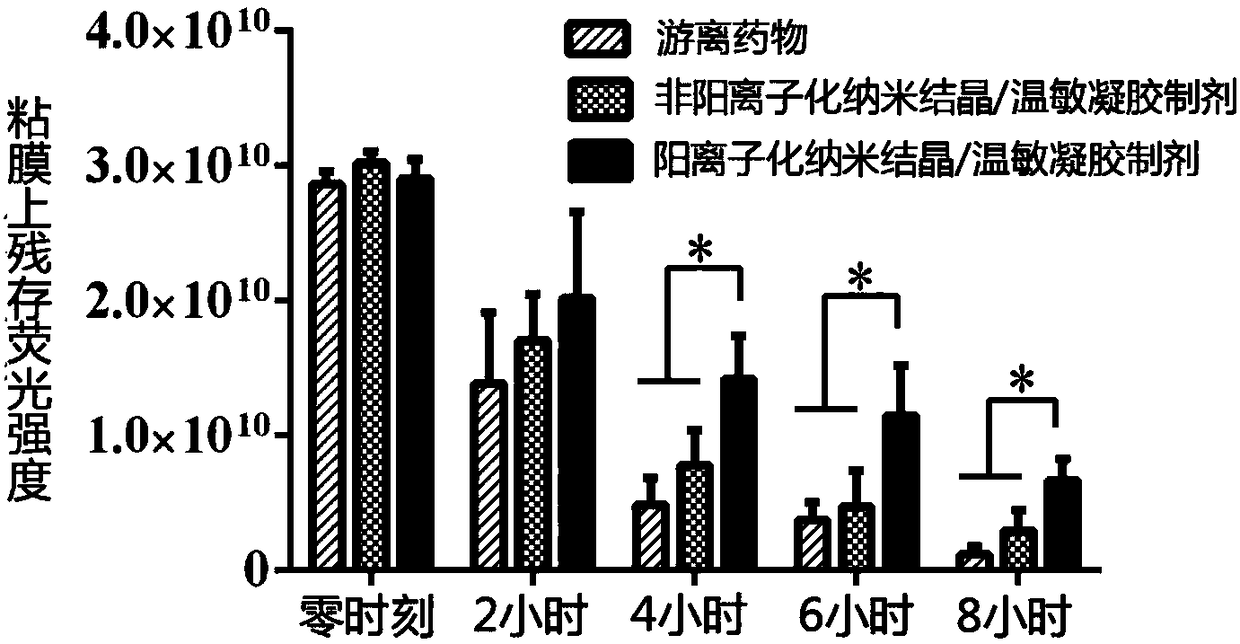
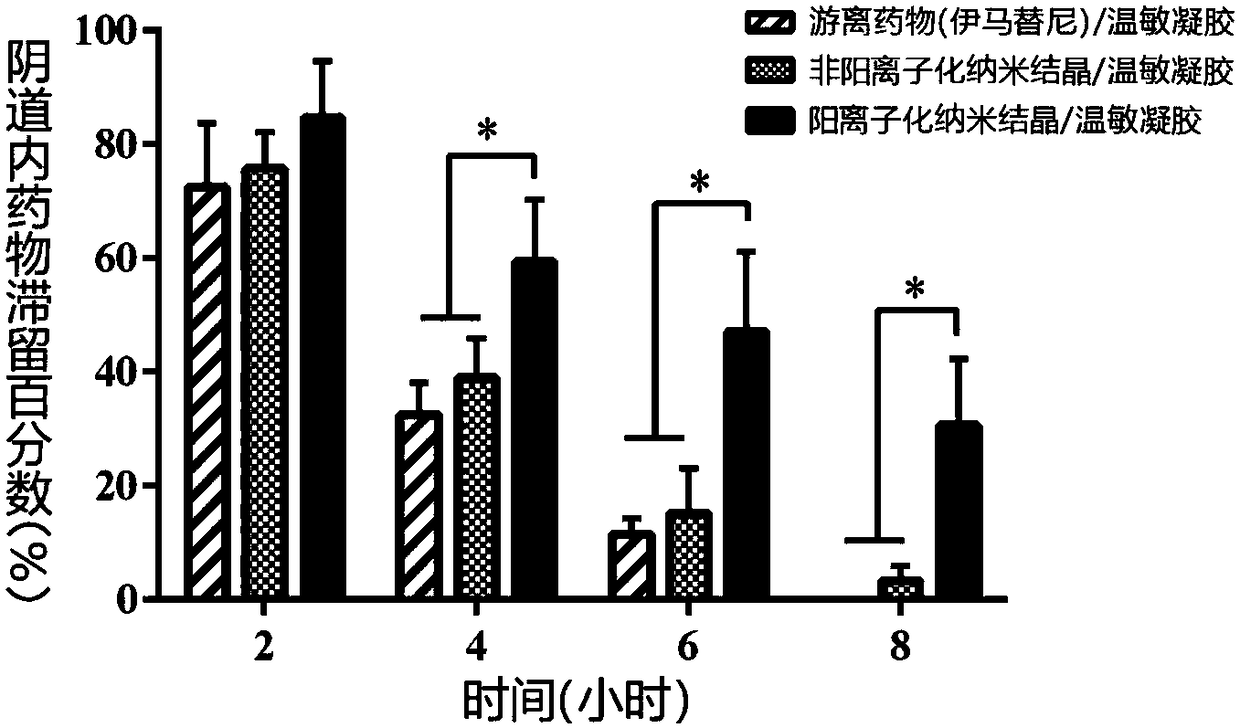
The invention belongs to the field of drug preparations and relates to a positively charged drug nanocrystal preparation capable of being used for mucosal drug delivery and featured with bioadhensionand a preparation method thereof. According to the positively charged drug nanocrystal preparation capable of being used for mucosal drug delivery and featured with bioadhension, by using a polydopamine coating technique, surface coating and positively charging of drug nanocrystals are realized, so that polydopamine coating layers adhere to the surfaces of the drug nanocrystals and amination modification is carried out on the surfaces of the drug nanocrystals; and therefore, the surfaces of the drug nanocrystals are rich in positive charges, and better adhesion and better stability to biological tissues are given to the drug nanocrystals. The positively charged drug nanocrystal preparation capable of being used for mucosal drug delivery and featured with bioadhension, provided by the invention, has the advantages that problems of insufficient stability, lack of bioadhension and the like of the nanocrystals in the prior art can be solved, the preparation can be used for multiple routesof mucosal drug delivery and can also be used for intravesical or surgical margin medication. Positive charges adhering to the surfaces of the drug nanocrystals in the positively charged drug nanocrystal preparation capable of being used for mucosal drug delivery and featured with bioadhension are beneficial for interaction between the nanocrystals and a biological mucosa or other tissues.
Owner:FUDAN UNIV
Glipizide tablet as well as preparation method and application thereof
ActiveCN105213335AEnhance drug releaseImprove bioavailabilityMetabolism disorderSulfonylurea active ingredientsAdhesiveDrug release rate
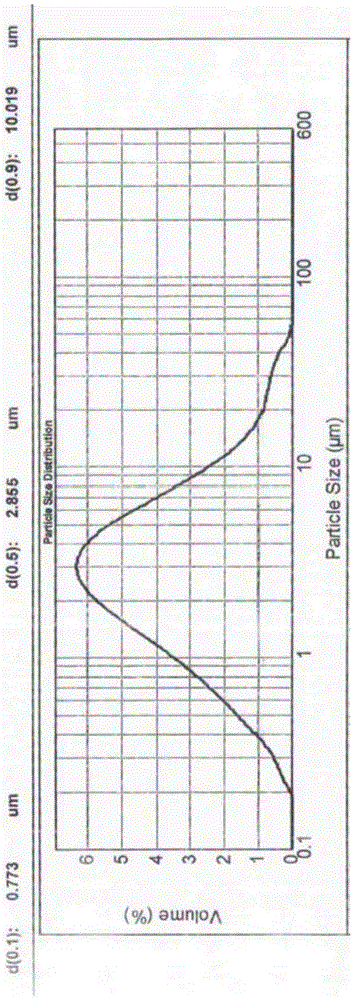
The invention discloses a glipizide tablet as well as a preparation method and an application thereof. The glipizide tablet comprises the following components in percentage by weight: 1-5% of glipizide with the particle diameter D50 being 50-100 micrometers, 1-10% of a solubilizer, 50-90% of a filler, 5-15% of a disintegrating agent, 0.1-5% of a lubricant, and a suitable amount of 2-15% adhesive, wherein glipizide is preferably glipizide with the particle diameter D50 being 50-75 micrometers. According to the invention, the active ingredient glipizide is micronized, so that the particle diameter of the bulk drug is reduced, meanwhile, the solubilizer is added to be matched with glipizide, so that the drug release rate is increased, further, the bioavailability is improved, and the quality of the product is improved.
Owner:KANGYA OF NINGXIA PHARMA
Co-amorphous substance of puerarin nicotinamide
The invention relates to a co-amorphous substance of puerarin nicotinamide, which can significantly improve dissolubility of an insoluble medicine, namely puerarin. The co-amorphous substance is in anamorphous state completely different from that of a puerarin crystal, and a melting point, an X-ray powder diffraction pattern, a DSC (differential scanning calorimetry) spectrogram and an infrared spectrum of the co-amorphous substance are different from those of the puerarin crystal. With the adoption of Cu-k alpha radiation, an X-ray powder diffraction spectrum expressed by 2 theta degree hasno sharp diffraction peak. A glass transition temperature of the substance is approximately 64.1 DEGC. A dissolving experiment result shows that the co-amorphous substance can increase an intrinsic dissolution rate of the puerarin by about 78 times and keep an oversaturation state higher than the dissolvability of the puerarin crystal for 24h.
Owner:CHINA PHARM UNIV
Valsartan dispersible tablet and preparation method thereof
ActiveCN104042580AGood dispersionImprove drug dissolutionPharmaceutical product form changePill deliveryValsartanDrug Dissolution
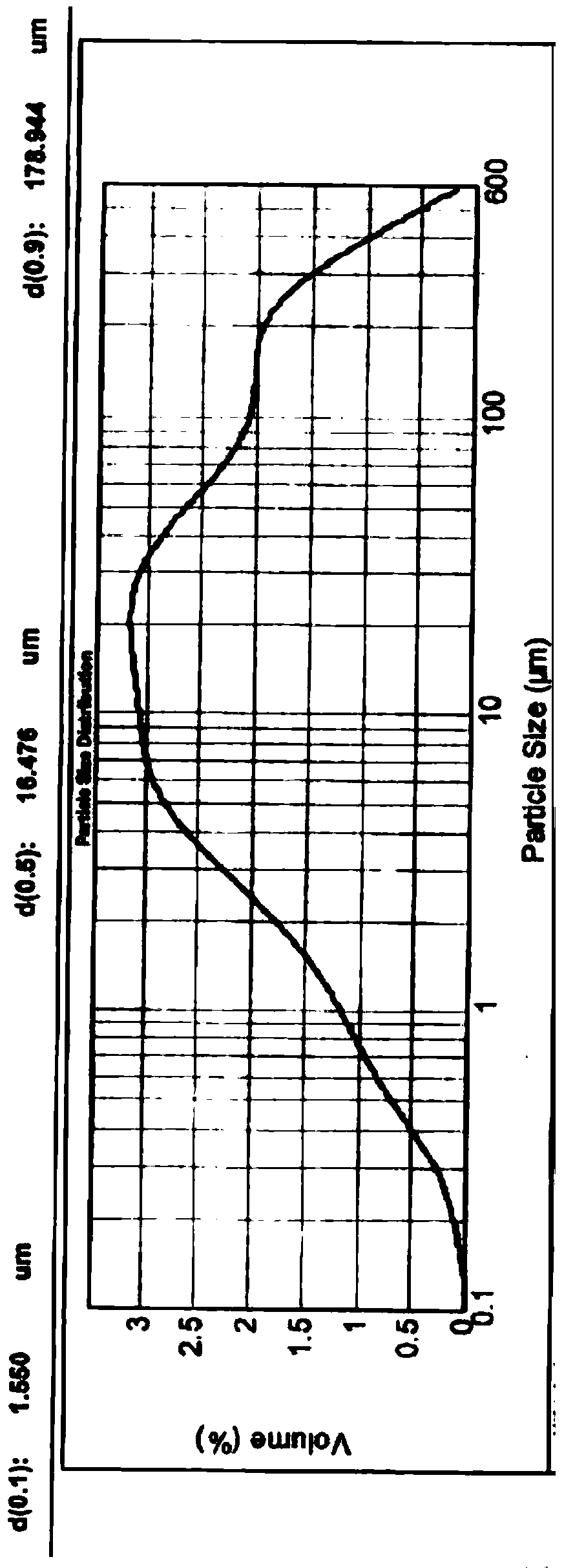
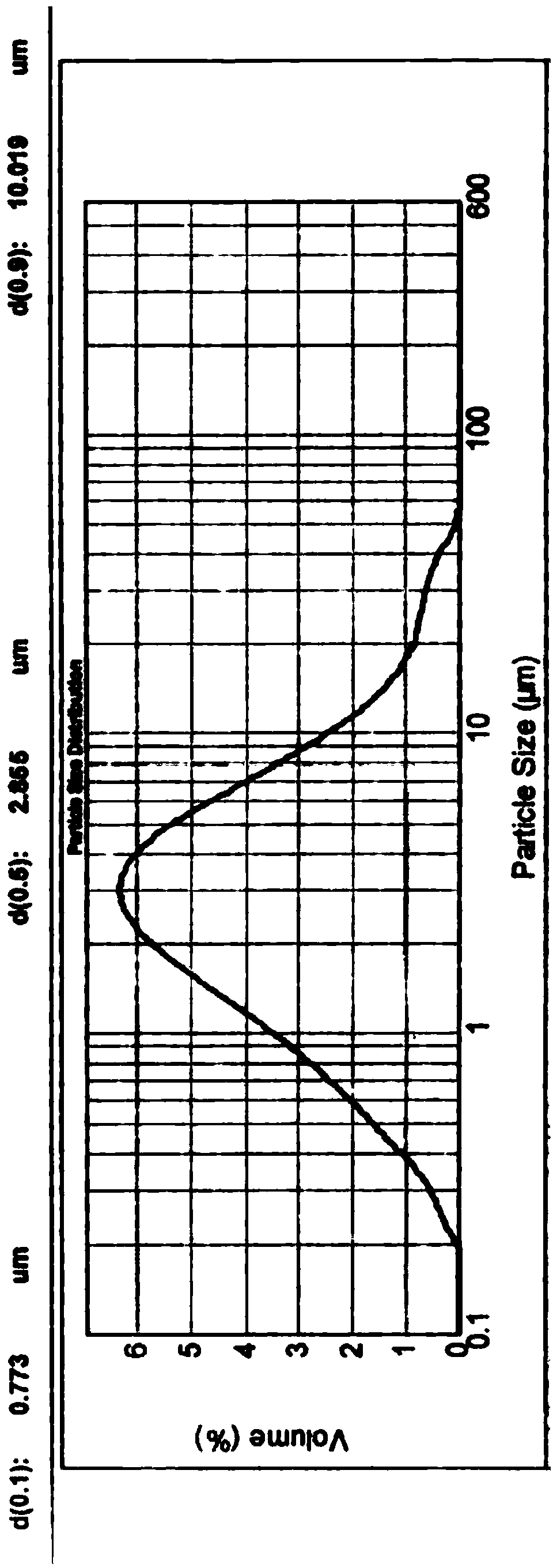
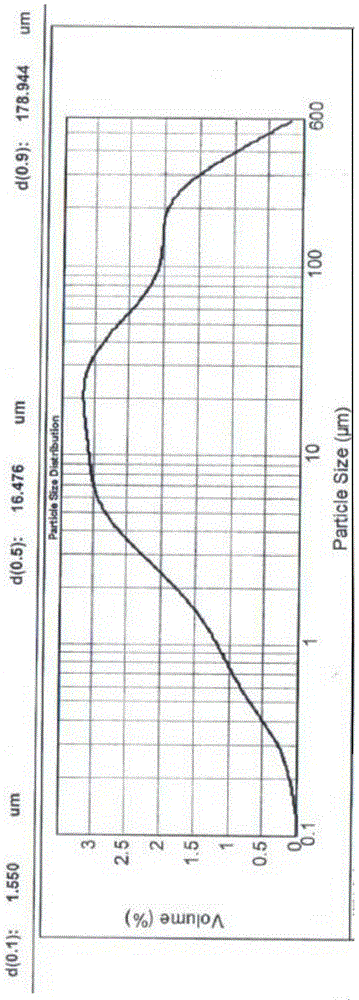
The invention relates to medicine field, and particularly relates to an angiotensin II receptor antagonist valsartan dispersible tablet and a preparation method thereof. The prescription of the product comprises pharmaceutical adjuvants acceptable in pharmacy like valsartan bulk drug, a filler, a disintegrant, an adhesive, flow aid, a lubricant, and corrigent, and the dispersible tablet is prepared by a wet granulation method. The valsartan bulk drug needs to be micronized, and the diameter 90 is less than 75mu m, preferably, the diameter 90 is less than 10mu m. The size distribution of the valsartan bulk drug directly affects the dispersibility of the dispersible tablet. The valsartan has polymorphy, different crystal forms of valsartan have different size distribution, and the dispersibility of the valsartan dispersible tablet is more different. The size distribution of the valsartan bulk drug is controlled by virtue of the micronization technology, the diameter 90 is less than 75mu m, preferably, when the diameter 90 is less than 10mu m, the difference of size distribution of different crystal forms of valsartan bulk drug is eliminated, the dispersibility of the valsartan dispersible tablet is improved obviously, the drug dissolution rate of the valsartan dispersible tablet is improved, and the product quality is controllable and is improved remarkably.
Owner:珠海润都制药股份有限公司
Micronized Chinese yew particles, compound for health bath obtained by using Chinese yew micronized particle and preparation method thereof
InactiveCN103202865ASimple processImprove crushing efficiencyPowder deliveryNervous disorderMicroparticleTraditional medicine
The invention relates to Chinese yew micronized particles, a compound for health bath obtained by using micronized Chinese yew particle and a preparation method thereof. The preparation method of the micronized Chinese yew particles comprises the following steps of: mixing Chinese yew bark and / or root and micronized solid auxiliary agent so as to form a solid mixture; and crushing the solid mixture in a micronized manner so as to form mixture particles with particle sizes D(90) being smaller than or equal to 15 microns. According to the micronized method disclosed by the invention, the dissolution rate of effective constituents of Chinese yew bark and root is greatly improved, the utilization ratio of Chinese yew bark and root is greatly improved, the adhesion and blockage conditions in crushing are also reduced, the medicine loss is few, and the crushing efficiency of micronized medicine can be obviously improved.
Owner:朱乃根
Functional beverage capable of preventing and treating cold and cough
InactiveCN104872773AEliminate side effectsImprove immunityYeast food ingredientsRespiratory disorderBiotechnologyBaical Skullcap Root
The invention discloses a functional beverage capable of preventing and treating cold and cough. The functional beverage is prepared from such raw materials in parts by weight as 18-22 parts of honeysuckle flower, 7-12 parts of baical skullcap root, 12-20 parts of dandelion, 4-8 parts of liquorice root, 5-7 parts of apricot kernel, 8-12 parts of perilla fruit, 80-100 parts of honey, 120-200 parts of white granulated sugar and 9000-11000 parts of water. The invention also discloses a preparation method of the functional beverage. The invention provides a natural pure traditional Chinese medicine functional beverage according to a reasonable formula; after the Chinese herbs are fermented, the toxic and side effects of the Chinese herbs are eliminated; the medicinal ingredients are promoted to be dissolved out by virtue of fermentation and wall-breaking; in addition, various vitamins and amino acids as well as trace elements contained in the raw materials can be dissolved out by virtue of Bayer in combination with fermentation with saccharomycetes and acetobacter xylinum, and no any hormone and chemical component need to be added; as a result, the functional beverage can be drank to achieve the effect of preventing and treating the cold and cough, and also has the effects of health care, body building, and organism immunity enhancement; the popularization of the functional beverage benefits the country.
Owner:杜沛雨
Valsartan tablet and preparation method thereof
InactiveCN107684549AWell mixedSmall particle sizePharmaceutical non-active ingredientsCoatingsValsartanMedicine
The invention discloses a valsartan tablet and a preparation method thereof, and belongs to the technical field of blood pressure lowering medicine. The valsartan tablet is prepared from the followingingredients in parts by weight: 320 parts of valsartan raw medicine, 350 to 400 parts of microcrystalline cellulose, 40 to 80 parts of polyvinylpolypyrrolidone, 10 to 15 parts of superfine silica gelpowder, 5 to 15 parts of magnesium stearate and 0.1 to 0.5 part of sodium dodecyl sulfate; the valsartan tablet is obtained from the raw materials through material mixing, blank sheet pressing, palletization, mixing, tabletting and coating. The valsartan tablet has the advantages that the raw material matching is scientific and reasonable; the absorption is fast; the preparation method is simple;the flow process is short; the production efficiency is greatly improved. By the production method, high-temperature operation is not needed; the influence of high-temperature environment on the product quality is avoided; the material adding, mixing, palletization and tabletting processes are reasonably controlled; the material fluidity is improved; the weight of each tablet is fully ensured; the product performance is good; the quality is stable; the requirement of scale production is met.
Owner:HUAREN PHARMACEUTICAL CO LTD
Antihypertensive functional beverage
InactiveCN104997115AEliminate side effectsImprove immunityYeast food ingredientsNatural extract food ingredientsBiotechnologyFermentation
The invention discloses an antihypertensive functional beverage. The antihypertensive functional beverage comprises the following raw materials in parts by weight: 12-18 parts of semen cassiae, 8-10 parts of cape jasmine, 12-18 parts of hawthorns, 14-16 parts of chrysanthemums, 8-12 parts of flos sophorae, 13-16 parts of cirsium setosum, 50-70 parts of honey, 120-170 parts of white granulated sugar, and 9000-11000 parts of water. The invention further discloses a preparation method of the antihypertensive functional beverage. According to the antihypertensive functional beverage disclosed by the invention, and through reasonable combination, a natural pure traditional Chinese medicine functional beverage is obtained. After the traditional Chinese medicines are fermented, the toxic and side effects of the traditional Chinese medicines are eliminated; through fermentation, cell walls are broken, the dissolution of valid ingredients of the traditional Chinese medicines is improved; saccharomyces baillii and acetobacter xylinum are combined for fermentation, so that various vitamins, amino acids and microelements in the raw materials can be dissolved out; the addition of hormones or chemical components is not needed. After being drunk, the functional beverage can achieve the effects of preventing and treating blood-pressure diseases; besides, the functional beverage has the efficacies of protecting health, building bodies and strengthening organic immunity. The popularization of the antihypertensive functional beverage benefits China and Chinese people.
Owner:杜圣艳
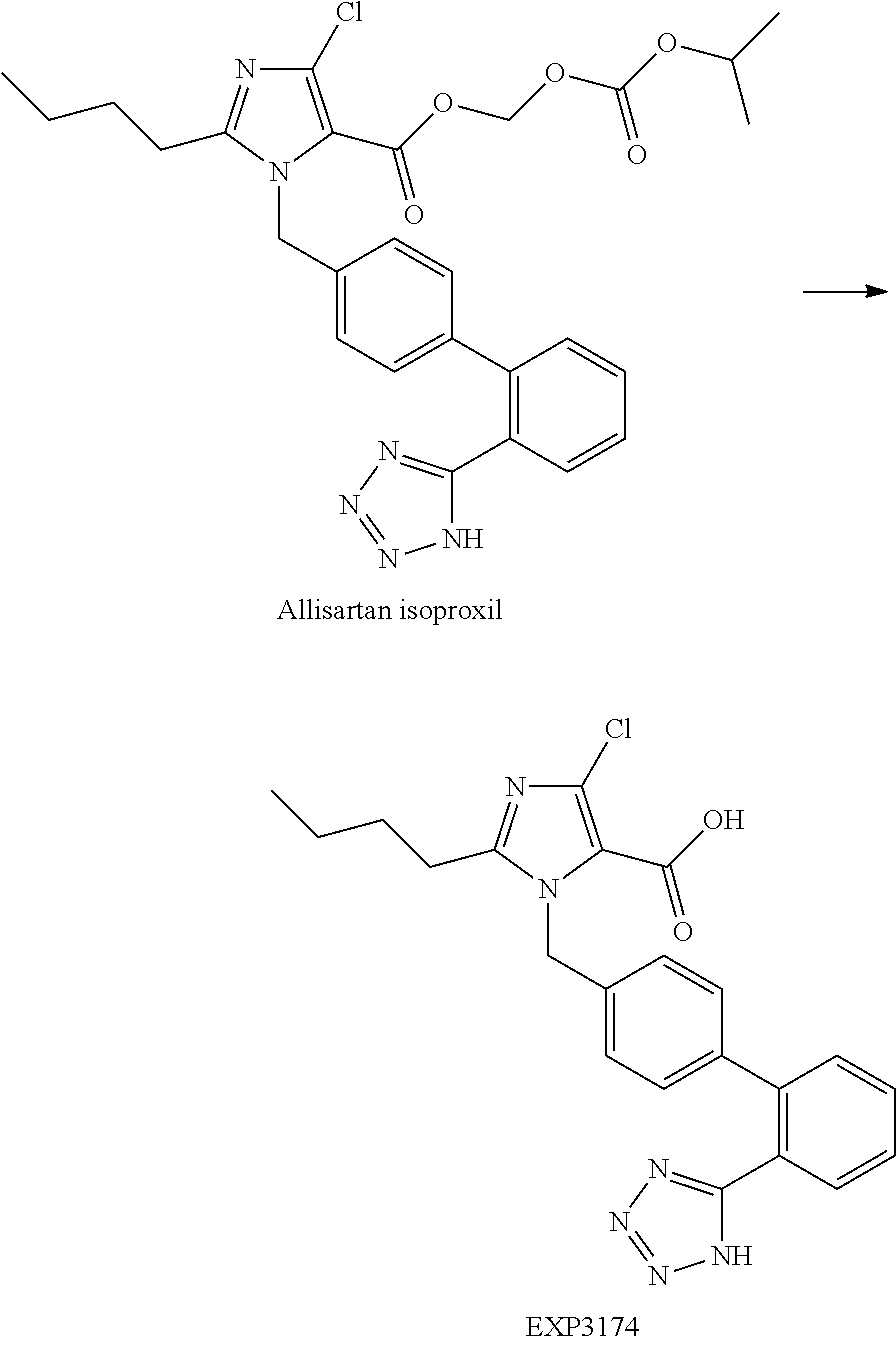
Allisartan isoproxil solid dispersion and pharmaceutical composition
InactiveUS20170135989A1High content of active ingredientsReduce the weight of the unitOrganic active ingredientsPowder deliveryPatient complianceMedicine
The present invention provides an allisartan isoproxil solid dispersion with high drug loading and stability. On that basis, it also provides an allisartan isoproxil pharmaceutical composition containing mentioned solid dispersion. Compared with available technologies, the mentioned allisartan isoproxil pharmaceutical composition is characterized by high stability, good dissolution performance, and improved patient compliance, etc.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
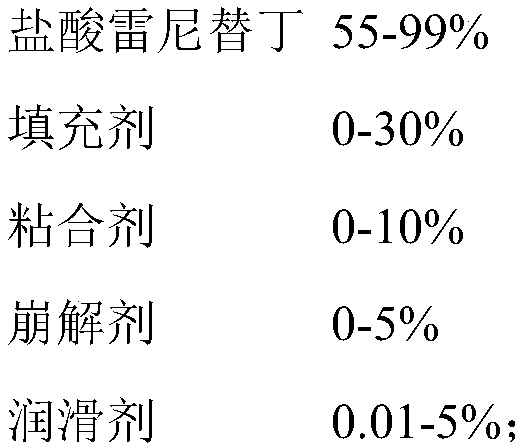
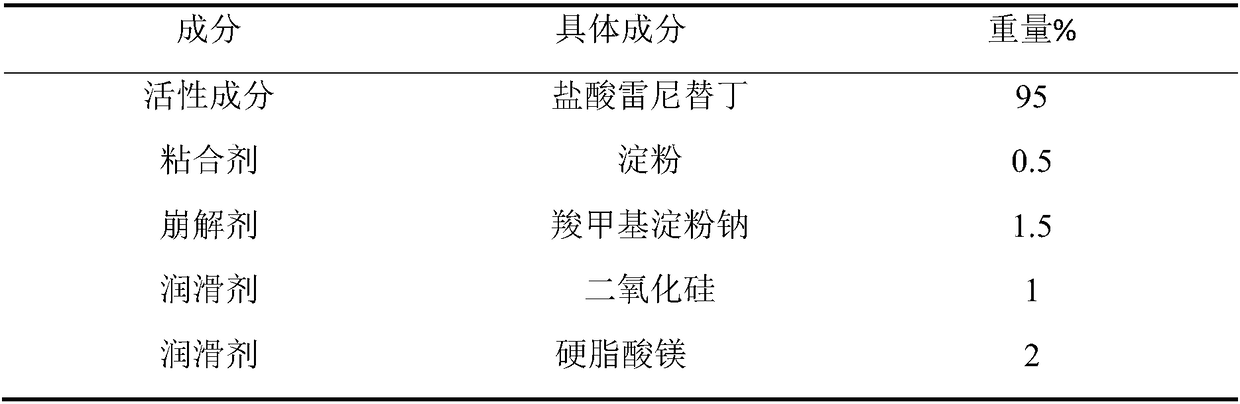
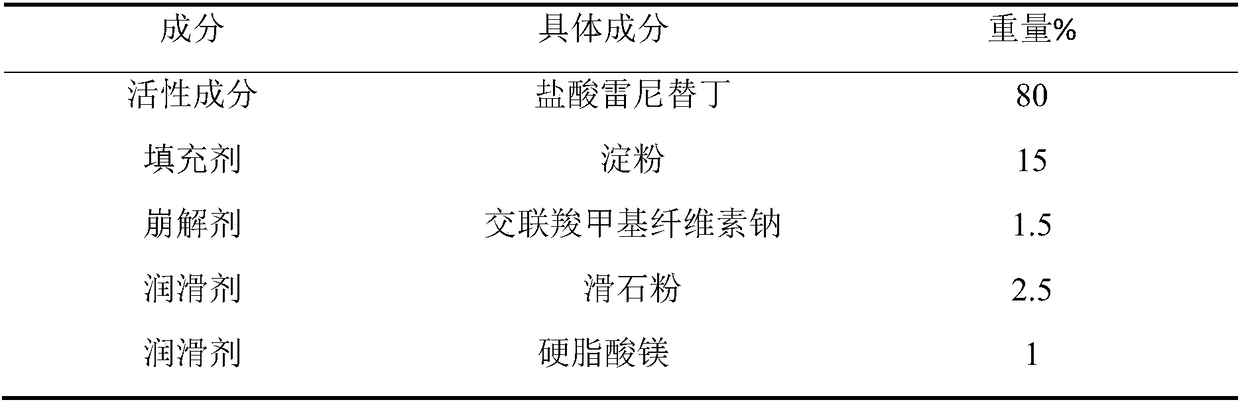
Industrial preparation method of ranitidine hydrochloride capsule
ActiveCN109010303AImprove drug dissolutionImprove bioavailabilityOrganic active ingredientsDigestive systemControllabilityMedicine
The invention relates to a preparation method of a ranitidine hydrochloride capsule. The ranitidine hydrochloride capsule mainly contains the following raw materials by weight percentage: 55-99% of ranitidine hydrochloride; 0-30% of a filler; 0%-10% of a binder; 0-5% of a disintegrant; and 0. 01-5% of a lubricant. The employed optimized prescription and process preparation method effectively improve the process robustness, controllability and feasibility; the production process of the ranitidine hydrochloride capsule is simplified, the production operation cycle is shortened, and the production efficiency is improved.
Owner:SUZHOU KELUN PHARMA RES CO LTD +1
Method for improving dissolution rate of cefquinome sulfate and method for detecting dissolution rate of cefquinome sulfate
InactiveCN111714501ASolve the problem of low dissolution rateImprove drug dissolutionAntibacterial agentsOrganic active ingredientsCEFQUINOME SULFATEDissolution
The invention discloses a method for improving the dissolution rate of cefquinome sulfate and a method for detecting the dissolution rate of the cefquinome sulfate. The method comprises the followingsteps of 1) mixing soybean oil for injection and ethyl oleate according to a ratio of 5: 5, heating mixed liquid to 120 DEG C under the protection of N2, keeping the temperature for 2 hours, and cooling the liquid to the room temperature; 2) heating the mixed liquid to 60 DEG C, adding BHT, performing stirring and melting, adding hydrogenated castor oil and span 60 when the liquid is cooled to 40DEG C, performing stirring for 30 minutes, and performing cooling to the room temperature; and 3) adding a prescription amount of the cefquinome sulfate after all medicinal auxiliary materials are added, pouring the cefquinome sulfate into a colloid mill, and performing grinding for 5-20 minutes in a manner of alternating circulating grinding and non-circulating grinding. The particle size of thecefquinome sulfate is changed through the colloid mill, so that the drug dissolution rate of the cefquinome sulfate is increased; and through particle size detection and dissolution rate analysis, after grinding is conducted for 15-20 min, the cefquinome sulfate with the particle size of 10 microns or below accounts for 80%, and the dissolution rate is the best.
Owner:杭州爱力迈动物药业有限公司
Solid cefpodoxime proxetil liposome preparation
InactiveCN102327217AImprove drug dissolutionSmall side effectsAntibacterial agentsOrganic active ingredientsLiposomeSide effect
The invention discloses a solid cefpodoxime proxetil liposome preparation and a preparation method thereof. The liposome is prepared with an active ingredient cefpodoxime proxetil and particular combination of beta-sitosterol, stearamide, cholesterol and Tween 80. The stability, dissolution and bioavailability of drugs can be greatly improved, stable and lasting effects can be achieved, and the curative effect is significant. The preparation product quality is improved, and toxic side effects are reduced.
Owner:HAINAN MEIDA PHARMA
Levamlodipine besylate tablet and preparation method thereof
ActiveCN103127018BLarge specific surface areaImprove drug dissolutionOrganic active ingredientsPill deliveryOrganic solventFluidized bed
The invention discloses a levamlodipine besylate tablet. The tablet is prepared by mixing a medicine carrying pellet containing levamlodipine besylate and pharmaceutically-accepted accessories and then carrying out direct compression, wherein the medicine carrying pellet is prepared by using the following method comprising the following steps of: weighting levamlodipine besylate and polyethylene glycol according to the weight ratio of 1: (4-8), then, dissolving levamlodipine besylate and polyethylene glycol into an organic solvent; and spraying the formed solution on a lactose pellet in a fluidized bed, coating and drying to obtain the medicine carrying pellet. The levamlodipine besylate raw material is coated on the outer layer of the lactose pellet and medicine is uniformly distributed on the surface of the pellet, so that the specific surface area of the medicine is greatly increased; and the raw material can be rapidly released, and thus, the medicine dissolution rate and the bioavailability of the levamlodipine besylate tablet are increased.
Owner:ZHEJIANG ANGLIKANG PHARMA
Naftopidil dispersible tablet and preparation method thereof
InactiveCN103006593AImprove clinical treatment effectFast disintegrationOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseTherapeutic effect
The invention discloses a naftopidil dispersible tablet and a preparation method thereof. The naftopidil comprises the following drugs in parts by weight: 12.5-30 parts of naftopidil, 0.5-2 parts of sweetening agent, 10-15 parts of talcum powder, 20-25 parts of sugar, 15-20 parts of explotab, 25-35 parts of cellulose, 17-25 parts of hydroxy propyl cellulose, 0.05-0.1 part of poloxamer and 0.5-1.0 part of magnesium stearate. The naftopidil dispersible tablet is used for treating prostatic hyperplasia, and has the advantages that the disintegration speed and the absorbing speed are quick, the drug dissolution rate and the bioavailability are high, the blood concentration can be stable quickly, and the clinical treatment effect of the prostatic hyperplasia can be improved.
Owner:NINGXIA DUOWEI PHARMA
Device and method for improving dissolution rate of medicine for treating parasitic infection
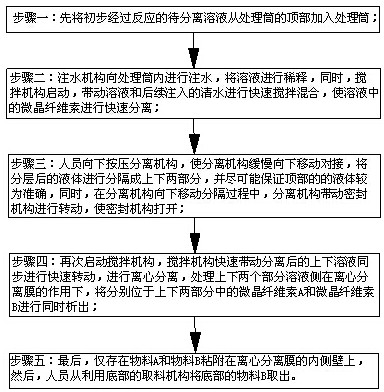
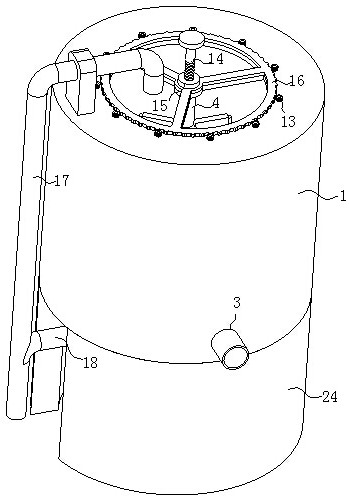
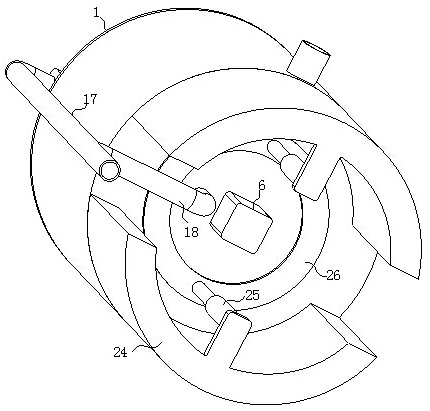
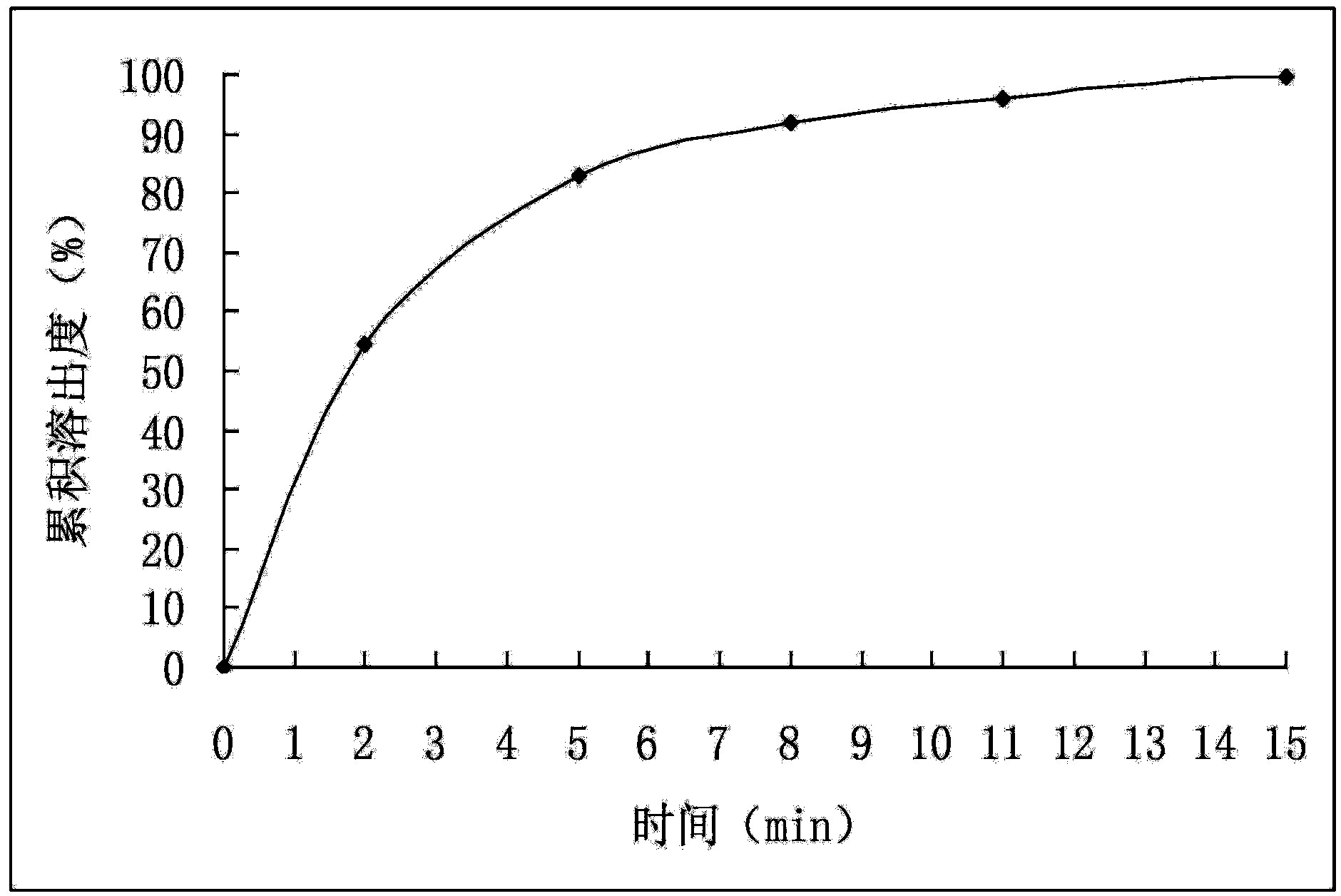
ActiveCN114028976ATimely separation and precipitationAvoid lostRotary stirring mixersTransportation and packagingPharmaceutical drugBiology
The invention discloses a device and a method for improving the dissolution rate of a medicine for treating parasitic infection, and belongs to the technical field of medicine dissolution. The device for improving the dissolution rate of the medicine for treating the parasitic infection comprises a treatment cylinder, and a centrifugal separation membrane is fixedly connected to the inner side of the treatment cylinder; the inner side of the treatment cylinder is connected with a sealing mechanism for supporting and sealing the centrifugal separation membrane in the diluting and mixing process; in each microcrystalline cellulose preparation process, the sealing mechanism and the stirring mechanism are matched with each other, so that a microcrystalline cellulose solution is layered more thoroughly, the microcrystalline cellulose is separated and separated out in time in cooperation with the separation mechanism, material taking is not needed, loss of the microcrystalline cellulose collected in the material taking process is avoided, and thus the separation effect of microcrystalline cellulose is improved, and the dissolution rate of drugs for treating parasitic infection is further improved.
Owner:JIANGXI CHUANGDAO ANIMAL HEALTH PROD
Taxus chinensis medicated bath formula and preparation method thereof
InactiveCN108079079AEasily volatileImprove drug dissolutionAntipyreticAnalgesicsCarthamusRemove blood
The invention discloses a taxus chinensis medicated bath formula and a preparation method thereof. The formula comprises folium artemisiae argyi, safflower carthamus, ligusticum chuanxiong hort, monkshood, radix cyathulae, cinnamon, radix clematidis, asarum and taxus chinensis essential oil powder. The preparation method comprises the preparation method of the taxus chinensis essential oil powderand the preparation method of taxus chinensis medicated bath powder, wherein the preparation method of the taxus chinensis essential oil powder comprises pretreatment, essential oil extraction and adsorption. The taxus chinensis medicated bath powder prepared by the preparation method is safe and non-toxic, enables the dissolution rate of beneficial components to be high by utilizing the volatility of the essential oil, and can promote metabolism, promote blood circulation and improve sleep. The principle is as follows: diffusion of the medicine components in the essential oil and warm stimulation can be conducted to a central nervous system through a touch receptor and a skin receptor, so that excitement and inhibition of the central pivot are ordered. The taxus chinensis medicated bath formula has the effects of activating main and collateral channels, dispelling cold and removing dampness as well as promoting blood circulation to remove blood stasis, and has a good effect of preventing and regulating rheumatism, rheumatoid as well as pain in the neck shoulder low-back.
Owner:重庆帕特企业管理咨询有限公司
Cefuroxime axetil tablets and preparation method thereof
The invention discloses a cefuroxime axetil tablets, which are prepared by mixing and directly tableting medicine-carrying pellets containing cefuroxime axetil, a filling agent and a lubricating agent, wherein the medicine-carrying pellets are prepared by the following steps of: weighing cefuroxime axetil and a disintegrating agent, dissolving or dispersing the cefuroxime axetil and the disintegrating agent into acetone to form a solution or suspension, spraying the solution or suspension into lactose pellets for coating in a fluidized bed, and drying to obtain the medicine-carrying pellets. According to the invention, the cefuroxime axetil raw material is coated on the outer layers of the lactose pellets, so that the medicine, namely the cefuroxime axetil can be uniformly distributed on the surfaces of the pellets, the specific surface area of the medicine is greatly increased, the raw cefuroxime axetil raw material can be rapidly released, and therefore the medicine dissolubility and the bioavailability are improved.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
New-type nano ibuprofen tablet and preparation method thereof
InactiveCN109394716AIncrease profitImprove drug dissolutionOrganic active ingredientsAntipyreticMedicineBiocompatibility Testing
The invention relates to a new-type nano ibuprofen tablet and a preparation method thereof, and relates to the technical field of drugs. The tablet comprises the following components: ibuprofen, a dispersing agent, a diluent and a binding agent. A preparation process for the tablet comprises the following steps: firstly enabling the ibuprofen to be uniformly dispersed in the dispersing agent and forming nano sol, after spray-drying, mixing with the diluent and the binding agent, pelletizing, to obtain the nano ibuprofen tablet. The tablet can be rapidly dispersed in water, and a dissolution rate is apparently higher than that of a tablet prepared by mixing and pressing a crude drug and same auxiliary materials. The auxiliary materials related in the tablet are in comply with pharmacopeia standards, biodegradable and good in biocompatibility. The method is simple in process, safe in operation, low in cost, and easy to realize industrial production of a ibuprofen nano-drug.
Owner:安徽东盛友邦制药有限公司
A kind of nanoparticle of insoluble drug and its preparation method and application
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Glipizide tablet and its preparation method and application
ActiveCN105213335BImprove drug dissolutionMetabolism disorderSulfonylurea active ingredientsDrug release rateAdhesive
The invention discloses a glipizide tablet as well as a preparation method and an application thereof. The glipizide tablet comprises the following components in percentage by weight: 1-5% of glipizide with the particle diameter D50 being 50-100 micrometers, 1-10% of a solubilizer, 50-90% of a filler, 5-15% of a disintegrating agent, 0.1-5% of a lubricant, and a suitable amount of 2-15% adhesive, wherein glipizide is preferably glipizide with the particle diameter D50 being 50-75 micrometers. According to the invention, the active ingredient glipizide is micronized, so that the particle diameter of the bulk drug is reduced, meanwhile, the solubilizer is added to be matched with glipizide, so that the drug release rate is increased, further, the bioavailability is improved, and the quality of the product is improved.
Owner:KANGYA OF NINGXIA PHARMA
Micronized Chinese yew particles, compound for health bath obtained by using Chinese yew micronized particle and preparation method thereof
InactiveCN103202865BSimple processImprove crushing efficiencyPowder deliveryNervous disorderMedicineBiology
The invention relates to Chinese yew micronized particles, a compound for health bath obtained by using micronized Chinese yew particle and a preparation method thereof. The preparation method of the micronized Chinese yew particles comprises the following steps of: mixing Chinese yew bark and / or root and micronized solid auxiliary agent so as to form a solid mixture; and crushing the solid mixture in a micronized manner so as to form mixture particles with particle sizes D(90) being smaller than or equal to 15 microns. According to the micronized method disclosed by the invention, the dissolution rate of effective constituents of Chinese yew bark and root is greatly improved, the utilization ratio of Chinese yew bark and root is greatly improved, the adhesion and blockage conditions in crushing are also reduced, the medicine loss is few, and the crushing efficiency of micronized medicine can be obviously improved.
Owner:朱乃根
Meisuoshuli suppository, and preparation method and use thereof
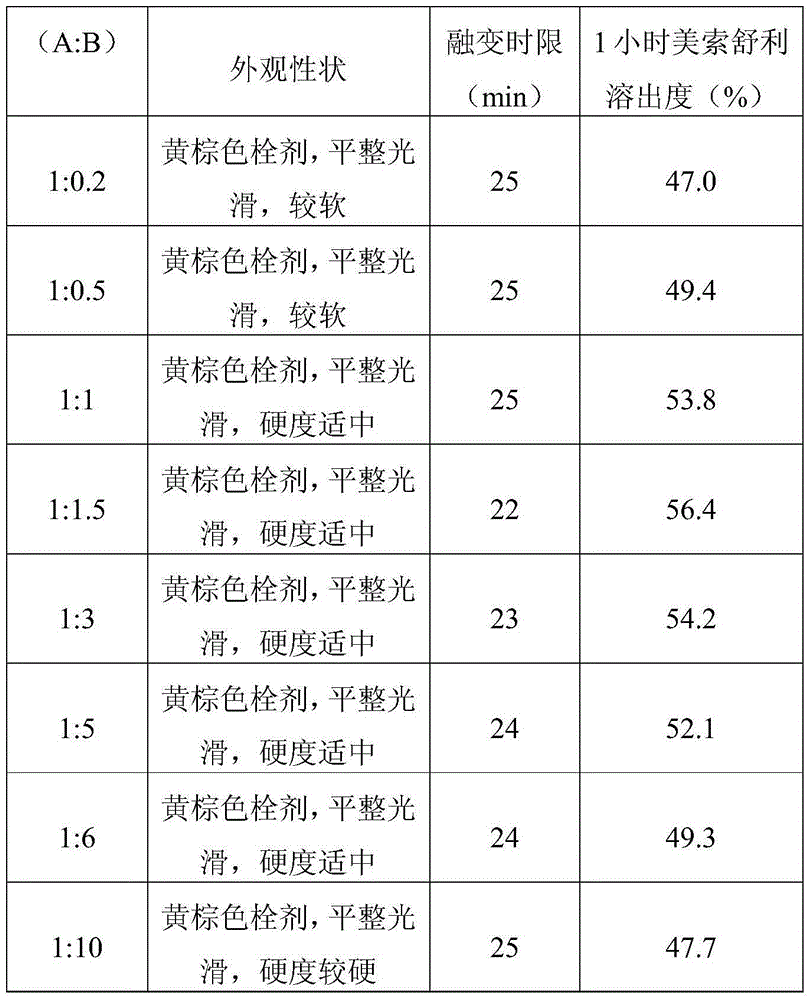
InactiveCN105434333AThe appearance meets the requirementsHardness meets the requirementsAntipyreticAnalgesicsPharmacyOral medication
The invention provides a Meisuoshuli suppository, and a preparation method and a use thereof. The Meisuoshuli suppository comprises Meisuoshuli and pharmaceutically acceptable auxiliary materials. The Meisuoshuli suppository has high dissolution rate and no irritation, has very good fever relieving, pain easing and inflammation preventing effects, has the advantages of large drug load, small administration frequency, simple use, low price, economy and practicality, and is very convenient for old people, children or unable oral administration patients to administrate.
Owner:WUHAN OPTICS VALLEY HUMANWELL BIO PHARMA +2
Functional beverage capable of decreasing blood sugar
InactiveCN104856046AEliminate side effectsImprove immunityFood ingredient functionsFood preparationBiotechnologyOfficinalis
The invention discloses a functional beverage capable of decreasing blood sugar. The functional beverage is prepared from the following raw materials in parts by weight: 12-18 parts of Chinese yam, 12-18 parts of radix polygonati officinalis, 8-10 parts of fructus gardenia, 8-10 parts of radix puerariae, 14-17 parts of lily, 8-12 parts of dark plum, 120-150 parts of honey and 9000-11000 parts of water. The invention further discloses a preparation method of the functional beverage. By virtue of a reasonable composition, the pure natural traditional Chinese medicine functional beverage is provided; after the traditional Chinese medicines are fermented, toxic and side effects of the traditional Chinese medicines are eliminated; by virtue of fermentation and wall breakage, the dissolution of pesticide effect is improved; furthermore, through fermentation through zygosaccharomyces bailii and acetobacter xylinum, various vitamins, amino acids and microelements contained in the raw materials are dissolved out, and no hormone and chemical components are added, so that the functional beverage has the effect of preventing and treating blood sugar diseases after being drunk as well as efficacies of building bodies and enhancing the immunity of the organism. By virtue of the popularization of the functional beverage, the nation and the people are benefited.
Owner:杜圣艳
A kind of valsartan dispersible tablet and preparation method thereof
ActiveCN104042580BSolve the uniformity of dispersionImprove dispersion uniformityPharmaceutical product form changePill deliveryValsartanDrug Dissolution
The invention relates to medicine field, and particularly relates to an angiotensin II receptor antagonist valsartan dispersible tablet and a preparation method thereof. The prescription of the product comprises pharmaceutical adjuvants acceptable in pharmacy like valsartan bulk drug, a filler, a disintegrant, an adhesive, flow aid, a lubricant, and corrigent, and the dispersible tablet is prepared by a wet granulation method. The valsartan bulk drug needs to be micronized, and the diameter 90 is less than 75mu m, preferably, the diameter 90 is less than 10mu m. The size distribution of the valsartan bulk drug directly affects the dispersibility of the dispersible tablet. The valsartan has polymorphy, different crystal forms of valsartan have different size distribution, and the dispersibility of the valsartan dispersible tablet is more different. The size distribution of the valsartan bulk drug is controlled by virtue of the micronization technology, the diameter 90 is less than 75mu m, preferably, when the diameter 90 is less than 10mu m, the difference of size distribution of different crystal forms of valsartan bulk drug is eliminated, the dispersibility of the valsartan dispersible tablet is improved obviously, the drug dissolution rate of the valsartan dispersible tablet is improved, and the product quality is controllable and is improved remarkably.
Owner:珠海润都制药股份有限公司
Method for improving dissolution rate of triclabendazole particles and dissolution rate detection method of triclabendazole particles
PendingCN113318079AImprove drug dissolutionSolve the problem of low dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsTriclabendazolePyrrolidinones
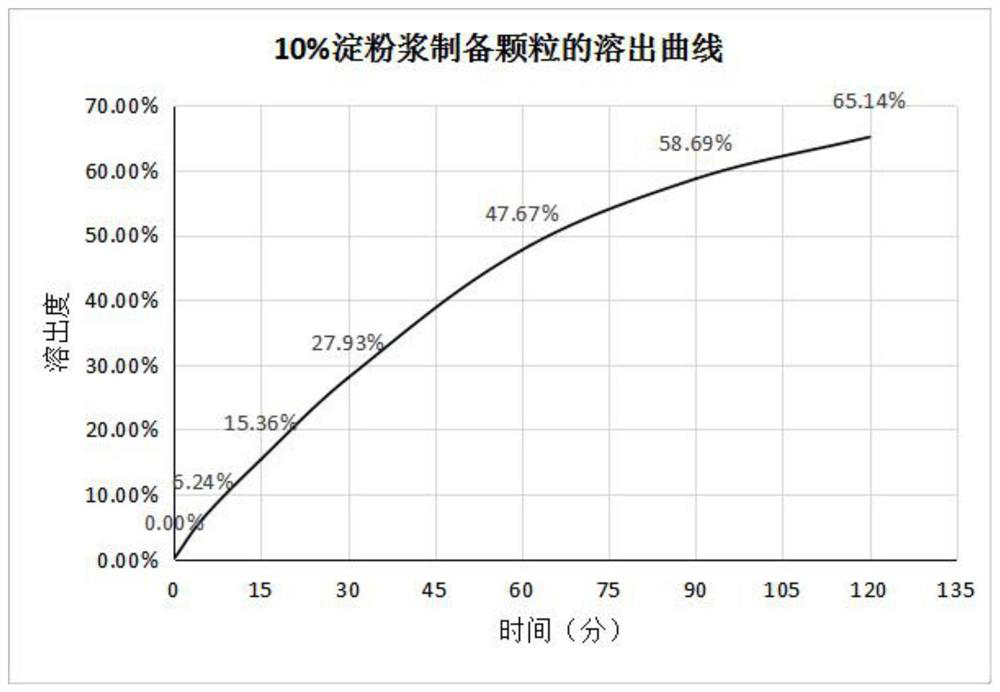
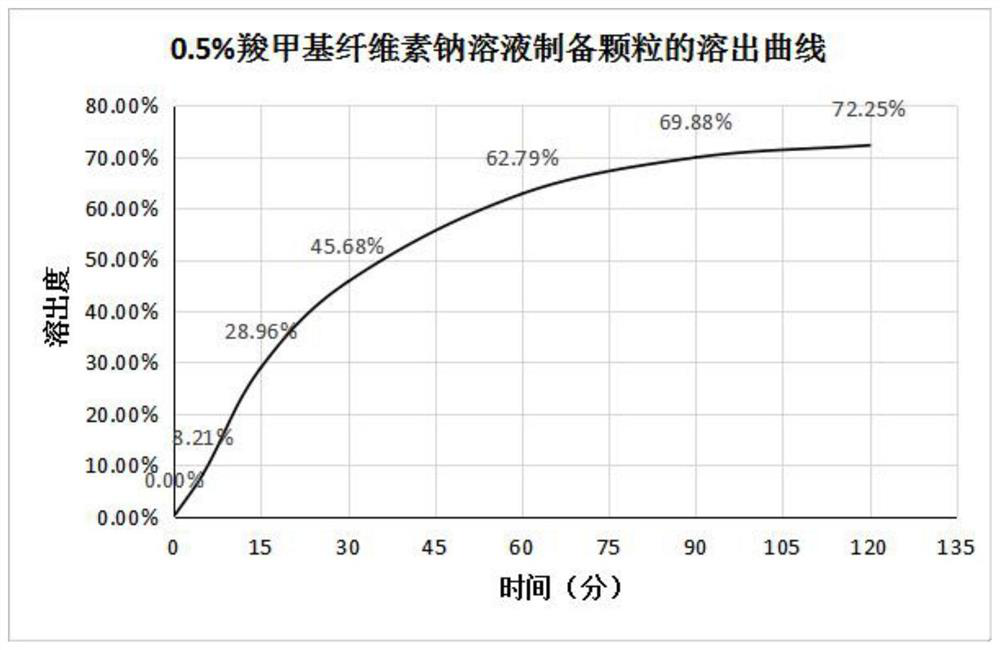
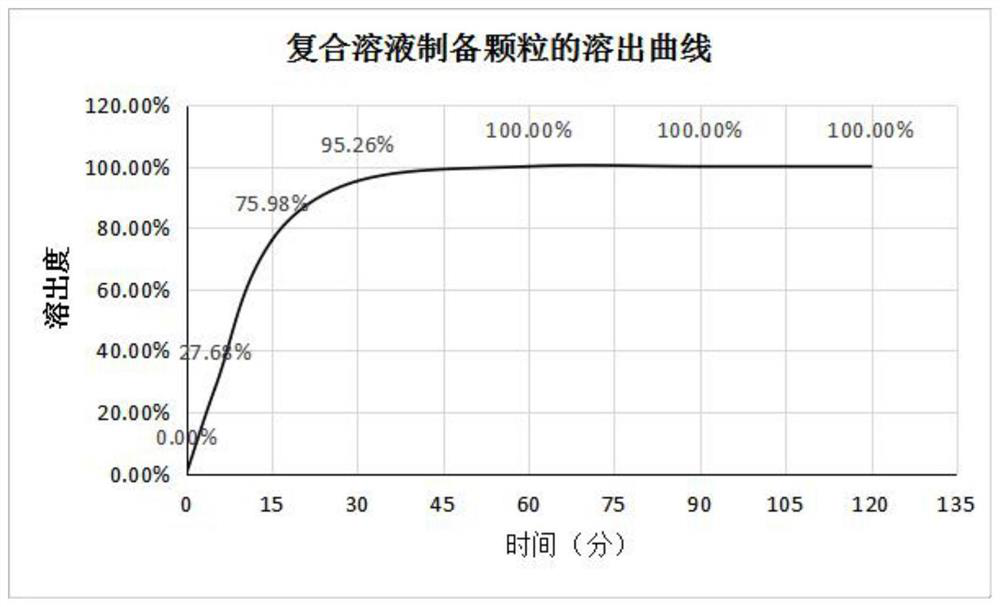
The invention discloses a method for improving the dissolution rate of triclabendazole particles and a dissolution rate detection method of the triclabendazole particles. The method comprises the following steps: firstly, mixing water, 95% ethanol, sorbitan monooleate and polyvinylpyrrolidone K30 according to a ratio of 3: 6: 0.4: 0.6, stirring and dissolving to obtain a clear and uniform composite solution for later use; then mixing triclabendazole, starch and glucose in a high-speed mixing pot according to a ratio of 1: 1: 8 for 5 minutes, slowly adding the mixture into the composite solution in the step S1 according to a ratio of 5% by adopting a method of stirring while adding, and then mixing for 5-10 minutes to prepare a soft material; and finally, feeding the soft material to a granulator, granulating with a 18-mesh screen, collecting, and drying in a drying oven. Compared with the prior art, the method for preparing the triclabendazole granules by using the composite solution has the advantages that the drug dissolution rate of the triclabendazole granules is improved, and the triclabendazole granules prepared from water, 95% ethanol, sorbitan monooleate and polyvinylpyrrolidone K30 according to the ratio of 3: 6: 0.4: 0.6 have the best dissolution rate.
Owner:JIANGXI BOLAI PHARMACY CO LTD
Functional beverage capable of preventing and treating rheumatism
InactiveCN104872761AEliminate side effectsImprove immunityFood ingredient functionsFood preparationBiotechnologyRheumatism
The invention discloses a functional beverage capable of preventing and treating rheumatism. The functional beverage is prepared from such raw materials in parts by weight as 10-15 parts of pawpaw, 8-10 parts of zaocys dhumnade, 8-10 parts of peach kernel, 13-18 parts of coix seed, 3-7 parts of cassia bark, 3-7 parts of dried ginger, 50-70 parts of honey, 120-200 parts of white granulated sugar and 9000-11000 parts of water. The invention also discloses a preparation method of the functional beverage. The invention provides a natural pure traditional Chinese medicine functional beverage according to a reasonable formula; after the Chinese herbs are fermented, the toxic and side effects of the Chinese herbs are eliminated; the medicinal ingredients are promoted to be dissolved by virtue of fermentation and wall-breaking; in addition, various vitamins and amino acids as well as trace elements contained in the raw materials can be dissolved out by virtue of Bayer in combination with fermentation with saccharomycetes and acetobacter xylinum, and no any hormone and chemical component need to be added; as a result, the functional beverage can be drank to achieve the effect of preventing and treating the rheumatism, and also has the effects of health care, body building, and organism immunity enhancement; the popularization of the functional beverage benefits the country.
Owner:杜圣艳
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com