Valsartan tablet and preparation method thereof
A valsartan tablet and prescription technology, applied in the field of valsartan tablet and its preparation, can solve problems such as unreasonable component matching, unstable product quality, complicated preparation process, etc., to avoid insufficient tablet weight, improve The effect of drug dissolution rate and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
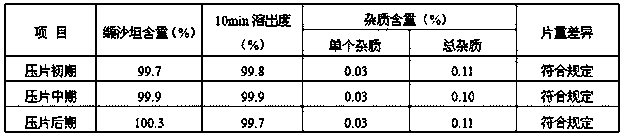
Examples
preparation example Construction
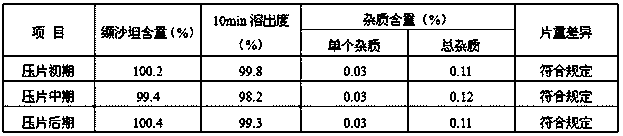
[0024] The preparation method of a kind of valsartan tablet of the present invention, comprises the following steps: (1) mixing material a, get respectively microcrystalline cellulose, magnesium stearate and sodium lauryl sulfate of 20-50% recipe quantity , and take the micropowder silica gel of prescription quantity, under the rotating speed of 5-15rpm, mix 3-10min, cross the sieve of 8-12 order, get the first mixture; Viketone and the remaining microcrystalline cellulose in the prescription amount are passed through a 16-22 mesh screen, and mixed at a speed of 8-12rpm for 8-12min to obtain the second mixture; c. Add the first mixture obtained in step a Add the second mixture obtained in step b, and mix at a speed of 8-15rpm for 10-20min to obtain the initial material; The material is pressed into embryo flakes of 7-15Kp; (3) granulation Add the embryo flakes obtained in step (2) into a pulverizer, and the mesh number of the pulverizer is 8-12 mesh to obtain primary grains; (...
Embodiment 1
[0030] Valsartan tablets are prepared by:
[0031] (1) Feed
[0032] Weigh 3.20Kg of valsartan, 3.88Kg of microcrystalline cellulose, 0.7Kg of crospovidone, 0.12Kg of micropowder silica gel, 0.1Kg of magnesium stearate, and 0.002Kg of sodium lauryl sulfate;
[0033] (2) Mixing
[0034] a. Take a part of microcrystalline cellulose, magnesium stearate and sodium lauryl sulfate of the above prescription, for example: take microcrystalline cellulose 1.552Kg, magnesium stearate 0.05Kg, sodium lauryl sulfate 0.001Kg , take the above-mentioned total prescription amount of micropowder silica gel, that is, 0.12Kg, add it to the mixer, 10rpm, mix for 5 minutes, add it to the comill sieve and sieve, the comill sieve is 0.062 inches, and the "F" type sieve, to obtain the first mixture;
[0035] B, get the valsartan raw material medicine of above-mentioned prescription quantity i.e. 3.20Kg and crospovidone i.e. 0.7Kg, and get the remaining microcrystalline cellulose in the above-mentione...
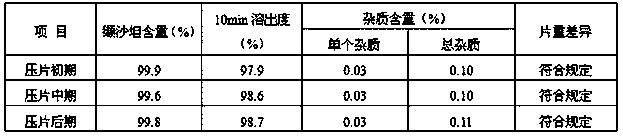
Embodiment 2
[0048] Valsartan tablets are prepared by:
[0049] (1) Feed
[0050] Weigh 3.20Kg of valsartan, 4.00Kg of microcrystalline cellulose, 0.55Kg of crospovidone, 0.15Kg of micropowder silica gel, 0.1Kg of magnesium stearate, and 0.001Kg of sodium lauryl sulfate;
[0051] (2) Mixing
[0052] a. Take a part of microcrystalline cellulose, magnesium stearate and sodium lauryl sulfate of the above prescription, for example: take microcrystalline cellulose 1.672Kg, magnesium stearate 0.05Kg, sodium lauryl sulfate 0.0005Kg , take the above-mentioned all prescription amount of micropowder silica gel, namely 0.15Kg, add it to the mixer, 10rpm, mix for 5min, put it into the comill sieve and sieve, the comill sieve is 0.062 inches, "F" type sieve, to get the first mixture;
[0053] B, get the valsartan crude drug of the above-mentioned prescription quantity i.e. 3.20Kg and crospovidone i.e. 0.55Kg, and get the remaining microcrystalline cellulose i.e. 2.328Kg in the above-mentioned treatme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com