Levamlodipine besylate tablet and preparation method thereof
A kind of technology of levamlodipine besylate and levamlodipine besylate, applied in the field of levamlodipine besylate tablet and preparation thereof, can solve problems such as complicated process, potential safety hazards, etc., and achieve simple preparation process and convenient operation , The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
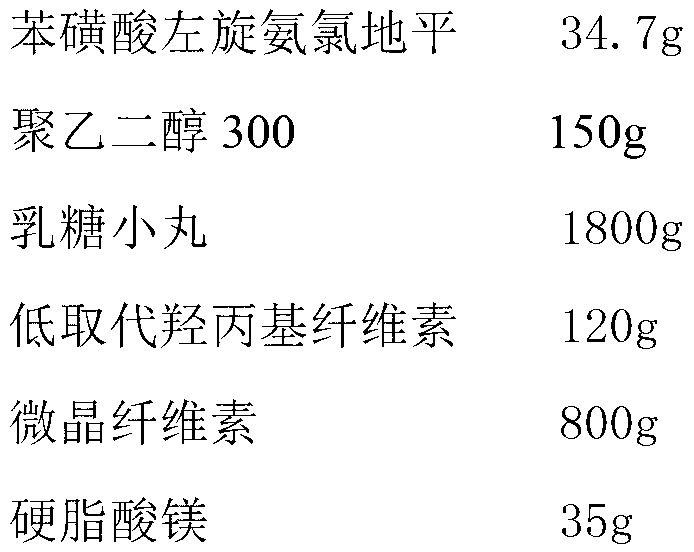
[0023] The preparation of embodiment 1 levamlodipine besylate tablets
[0024]
[0025] Preparation Process:
[0026] (1) Dissolve levamlodipine besylate and polyethylene glycol 300 in 1L of ethanol, spray the formed solution onto lactose pellets in a fluidized bed, coat them and dry them to obtain drug-loaded pellets;
[0027] (2) Mix uniformly the drug-loaded pellets obtained in step (1) with low-substituted hydroxypropyl cellulose, microcrystalline cellulose and magnesium stearate, and directly compress into tablets to obtain levamlodipine besylate tablets.
Embodiment 2
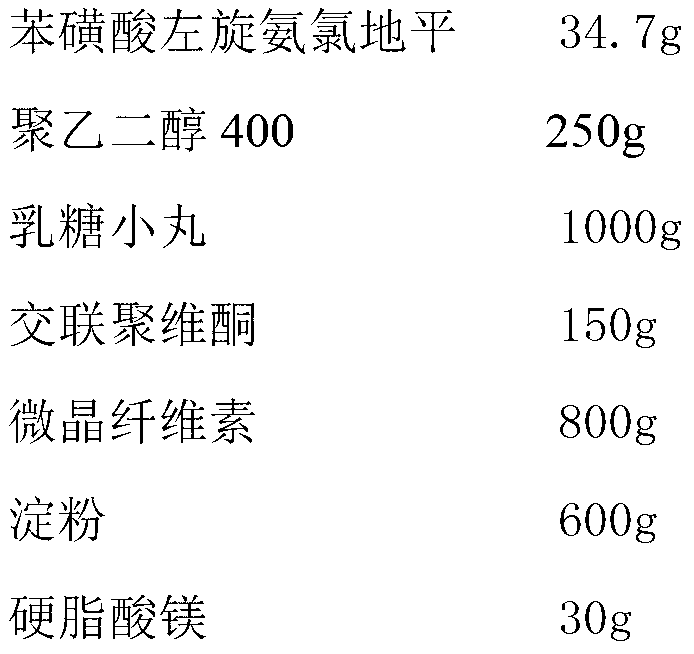
[0028] The preparation of embodiment 2 levamlodipine besylate tablets
[0029]
[0030] Preparation Process:
[0031] (1) Dissolve levamlodipine besylate and polyethylene glycol 400 in 1L of ethanol, spray the formed solution onto lactose pellets in a fluidized bed, coat them and dry them to obtain drug-loaded pellets;
[0032] (2) Mix uniformly the drug-loaded pellets obtained in step (1) with crospovidone, microcrystalline cellulose, starch and magnesium stearate, and directly compress into tablets to obtain levamlodipine besylate tablets.
Embodiment 3
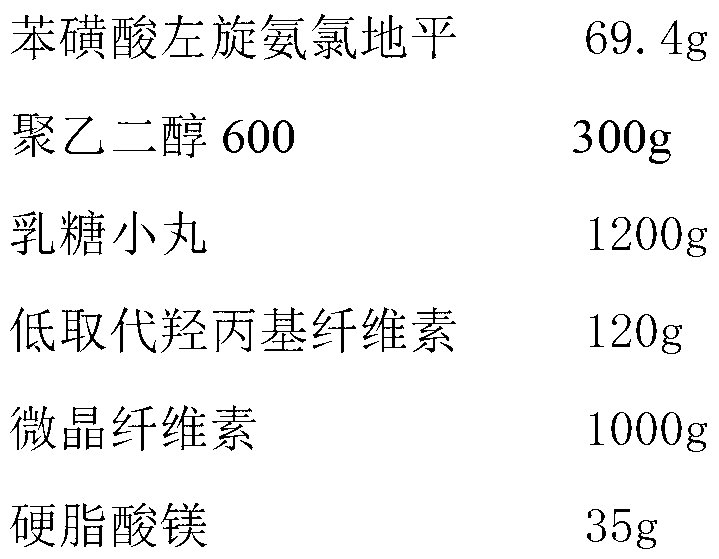
[0033] The preparation of embodiment 3 levamlodipine besylate tablets
[0034]
[0035] Preparation Process:
[0036] (1) Dissolve levamlodipine besylate and polyethylene glycol 600 in 1L of ethanol, spray the formed solution on lactose pellets in a fluidized bed, coat them and dry them to obtain drug-loaded pellets;
[0037] (2) Mix uniformly the drug-loaded pellets obtained in step (1) with low-substituted hydroxypropyl cellulose, microcrystalline cellulose and magnesium stearate, and directly compress into tablets to obtain levamlodipine besylate tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com