Positively charged drug nanocrystal preparation and preparation method thereof
A nano-crystal, positively charged technology, applied in pharmaceutical formulations, aerosol delivery, medical preparations with non-active ingredients, etc., can solve the problems of insufficient stability of nano-crystals, lack of bioadhesion, etc., and improve drugs. The effect of dissolution, prolongation of residence time and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
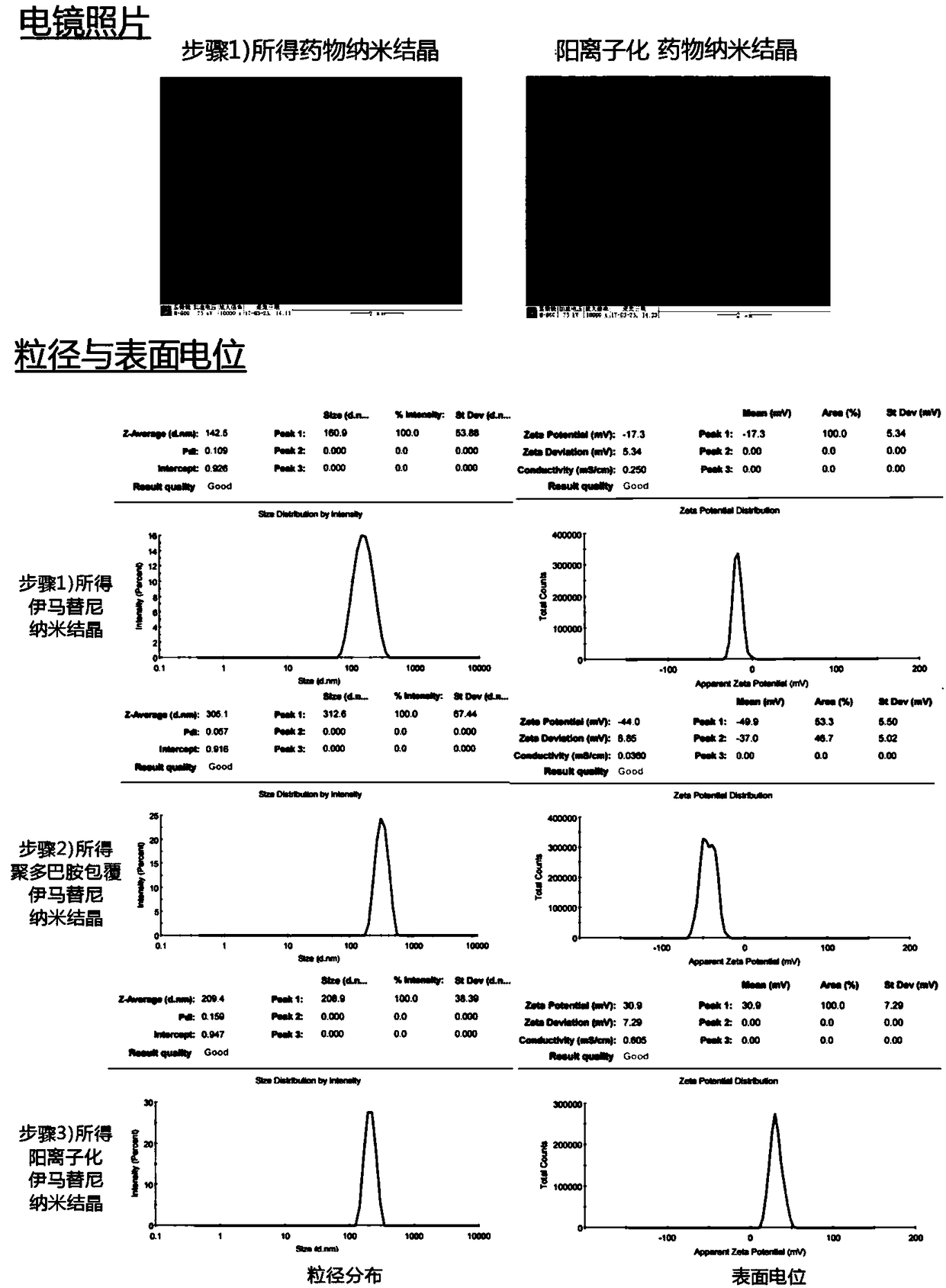
[0035] Example 1 Preparation and characterization of imatinib cationized nanocrystals
[0036] Preparation
[0037] Step 1: Accurately weigh imatinib (3.35 mg), polyethylene glycol α-tocopheryl succinate (3.7 mg) and citric acid (7.6 mg), add 2 mL of acetonitrile and stir to dissolve, and place in a pear-shaped bottle film formation by rotary evaporation at room temperature. Add 5mL sodium bicarbonate aqueous solution (3mg / mL), shake and hydrate to obtain imatinib nano-crystal suspension;
[0038] Step 2: adjust the pH to 7.5 with dilute hydrochloric acid, add an appropriate amount of dopamine hydrochloride (5 mg), stir electromagnetically at room temperature, react for 30 minutes, centrifuge at 10,000 rpm for 5 minutes, discard the supernatant, and disperse the precipitate with pure water to obtain the cationic ima Tiny nano crystal suspension;
[0039] Step 3: Put in 10 μL of N-(tert-butoxycarbonyl)-1,2-diaminoethane, stir electromagnetically at room temperature, react ...
Embodiment 2
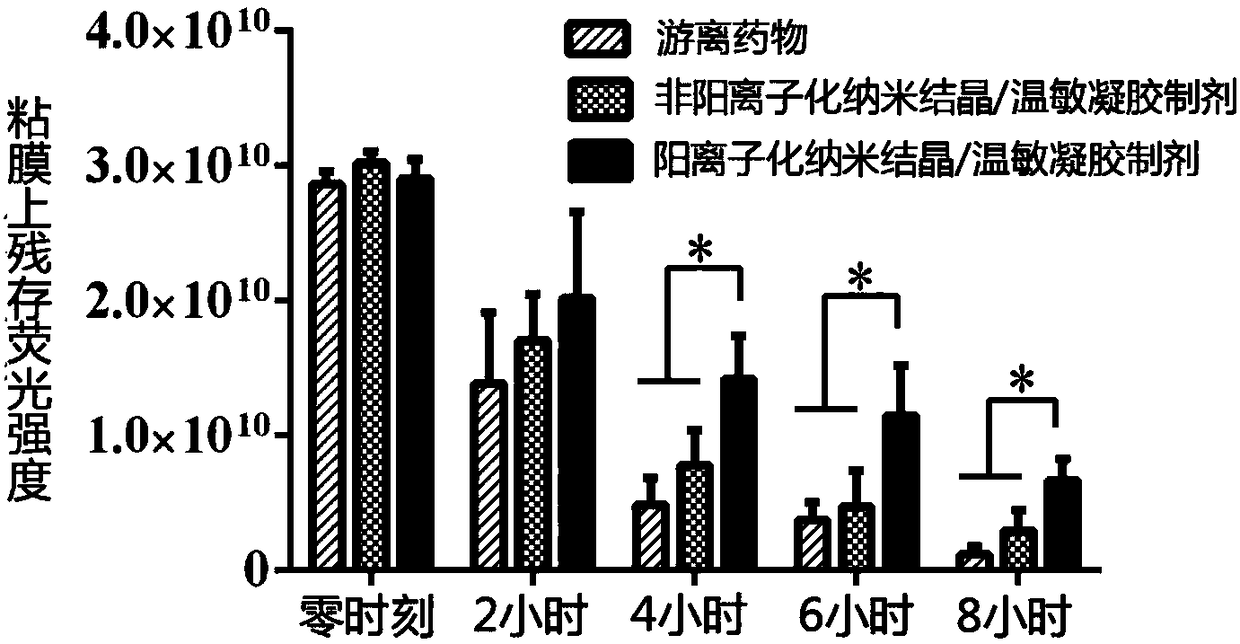
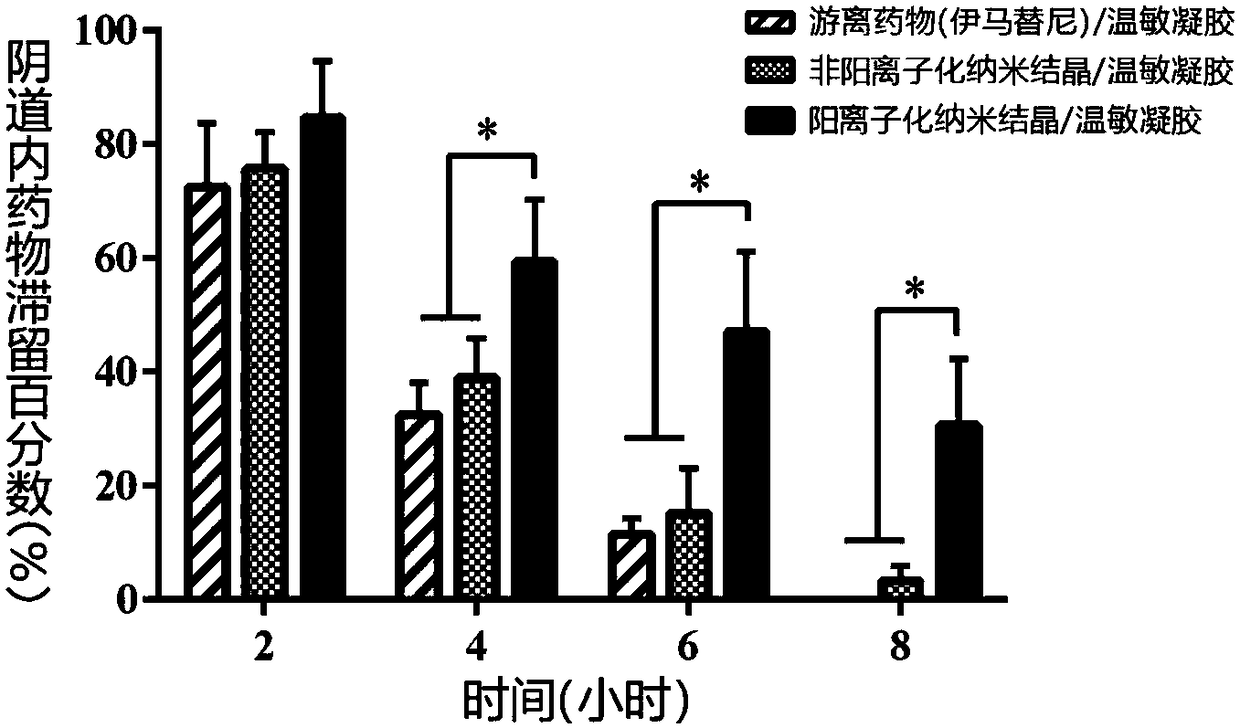
[0047] Example 2 Preparation and Characterization of Imatinib Cationic Nanocrystalline / Temperature Sensitive Gel Preparation
[0048] Preparation
[0049] Step 1: Same as Example 1.
[0050] Step 2: Same as Example 1.
[0051] Step 3: Put in 10 μL of N-(tert-butoxycarbonyl)-1,2-diaminoethane, stir electromagnetically at room temperature, react for 4 hours, add an appropriate amount of hydrochloric acid to adjust the pH to 2, terminate the reaction and remove the protecting group on the amino group , centrifuged at 10,000rpm for 5 minutes, discarded the supernatant, dispersed the precipitate with 0.77mL acetate buffer (pH 5.5, 100mM), then added 0.13g poloxamer 407, and stirred electromagnetically under ice bath until the solid was completely Dissolve, that is.
[0052] Finished product test
[0053] The gelation temperature of the obtained imatinib cationized nano-crystal / temperature-sensitive gel preparation is 28-30° C., it is liquid when the gelation temperature is ...
Embodiment 3
[0056] Example 3 Preparation of Curcumin / Imatinib Composite Cationic Nanocrystalline Preparation / Temperature Sensitive Gel Preparation
[0057] Preparation
[0058] Step 1: Accurately weigh curcumin (1.25mg), imatinib (1.65mg), polyethylene glycol α-tocopheryl succinate (3.7mg) and citric acid (7.6mg), add 2mL of acetonitrile and stir Dissolved, placed in a pear-shaped bottle, and rotatively evaporated at room temperature to form a film. Add 5mL sodium bicarbonate aqueous solution (3mg / mL), shake and hydrate to obtain curcumin / imatinib composite nanocrystal suspension;
[0059] Step 2: adjust the pH to 7.5 with dilute hydrochloric acid, add an appropriate amount of dopamine hydrochloride (5 mg), stir electromagnetically at room temperature, react for 30 minutes, centrifuge at 10,000 rpm for 5 minutes, discard the supernatant, and disperse the precipitate with pure water;
[0060] Step 3: Put in 10 μL of N-(tert-butoxycarbonyl)-1,2-diaminoethane, stir electromagnetically at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com