Valsartan dispersible tablet and preparation method thereof
A technology of dispersible tablets and valsartan, which is applied in the field of valsartan dispersible tablets and its preparation, can solve problems such as product dispersibility differences, and achieve the effects of improving dispersibility, simple operation, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 different particle size distribution valsartan crude drug
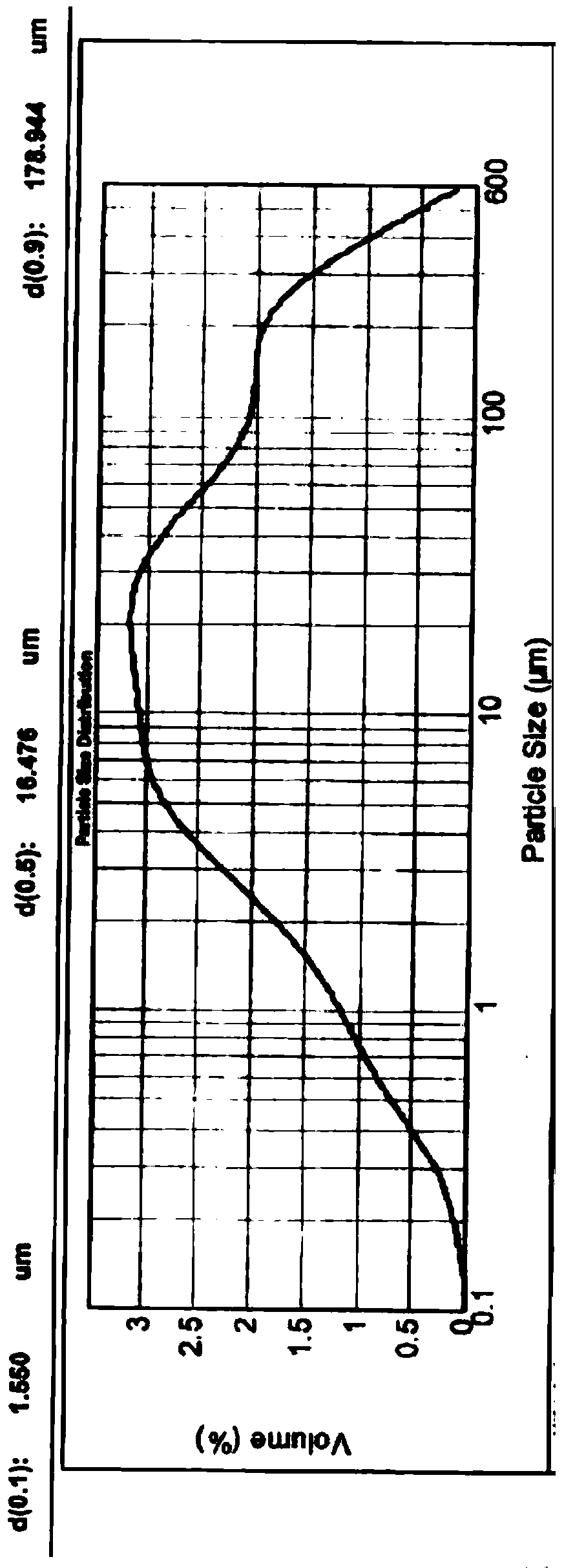
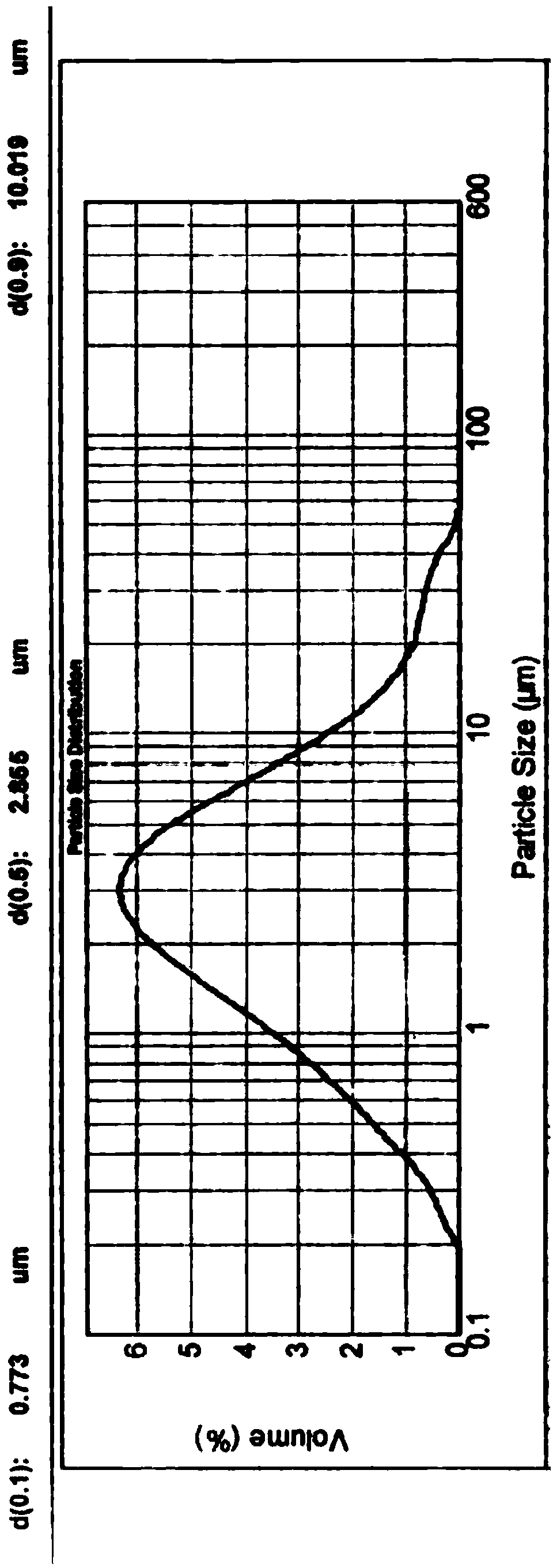
[0050] Get 5 kilograms of valsartan bulk drug without crushing after drying, be divided into 5 parts on average, each part of 1 kg, and do the following processing respectively: a portion of valsartan bulk drug crosses 20 mesh sieves to obtain valsartan bulk drug A; A portion of valsartan raw material passed through a 24-mesh sieve to obtain valsartan raw material B; another 3 parts of valsartan raw material were placed in a jet mill (model: QYF-100, manufacturer: Kunshan Miyou Powder Equipment Engineering Co., Ltd. company) carried out micronization for 15 minutes, 30 minutes, and 1 hour respectively, and obtained valsartan crude drug C, D, and E respectively. We used a Malvern particle size analyzer to detect the particle size distribution of the above five valsartan raw materials by dry method, and also tested their bulk density and compactness. The relevant parameters are sho...
Embodiment 2
[0054] The prescription screening and preparation of embodiment 2 valsartan dispersible tablets
[0055] Get the valsartan bulk drug E (D90=7.117um) through airflow pulverization, feed the ingredients according to the prescription listed in Table 2 respectively, mix in the wet granulator for 5 minutes, and gradually add the binder solution of 5% PVP K30 , and then add an appropriate amount of purified water, granulate for about 3 minutes, until the wet granules "hold into a ball, lightly knead and then disperse", pass through a 24-mesh sieve, put the wet granules in a tray, and spread them into a size of no more than 2cm. Thickness, dry in an oven at 55°C until the water content does not exceed 3%, and collect. The dried granules are granulated through 20 meshes, and mixed with additional crospovidone (PVPP), micronized silica gel and magnesium stearate for 15 minutes. Tablet compression, die ф8cm, each valsartan dispersible tablet contains 80mg of valsartan, and the hardness...
Embodiment 3
[0060] The valsartan crude drug of embodiment 3 different particle size distributions prepares valsartan dispersible tablet
[0061] Get each 80g of valsartan crude drug A, B, C, D, E of different particle size distributions, press the feed intake of raw and auxiliary materials listed in Table 3 prescription 6, prescription 7, prescription 8, prescription 9 and prescription 10 respectively, in wet Mix in the French granulator for 5 minutes, gradually add 5% PVP K30 binder solution, and then add an appropriate amount of purified water, and granulate for about 3 minutes until the wet granules "come into a ball by hand, and then disperse when gently pinched". Pass through a 24-mesh sieve, place the wet granules on a tray, spread out to a thickness of no more than 2cm, and dry in an oven at 55°C until the moisture content does not exceed 3%, then collect. The dried granules are granulated through 20 meshes, and mixed with additional crospovidone (PVPP), micronized silica gel and m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com