Cefuroxime axetil capsule and preparation method thereof
A technology of cefuroxime axetil and capsules, which is applied in the field of cefuroxime axetil capsules and its preparation, can solve the problems of low dissolution rate of granules, great influence on dissolution rate, poor fluidity, etc., so as to improve bioavailability and drug dissolution Degree, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Preparation Process:
[0024] (1) Dissolve cefuroxime axetil in 1 liter of acetone, add crospovidone, stir, grind in a ball mill, control the particle size of the suspension D80<25 microns, and place the formed suspension in a fluidized bed Spray and coat the lactose pellets to obtain drug-loaded pellets.
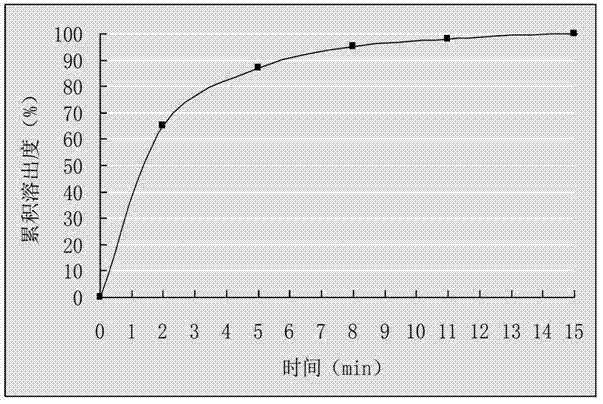
[0025] (2) Mix the drug-loaded pellets obtained in step (1) evenly with talcum powder, and put them into capsule shells to obtain cefuroxime axetil capsules. Get 6 cefuroxime axetil capsules that this example prepares, put into the sedimentation blue respectively, take 0.07mol / L hydrochloric acid solution 900mL as dissolution medium, rotating speed 55r / min, operate according to law, at 0, 2, 5, 8, 11 , 15min, 5mL samples were taken for measurement and the cumulative dissolution percentage was calculated. Take the time t as the abscissa, and the average cumulative dissolution percentage is plotted as the ordinate, and the results are shown in figure 1 ....
Embodiment 2
[0027]
[0028] Preparation Process:
[0029] (1) Dissolve cefuroxime axetil in 1 liter of acetone, add crospovidone, stir, grind in a ball mill, control the particle size of the suspension D80<25 microns, and place the formed suspension in a fluidized bed Spray and coat the lactose pellets to obtain drug-loaded pellets.
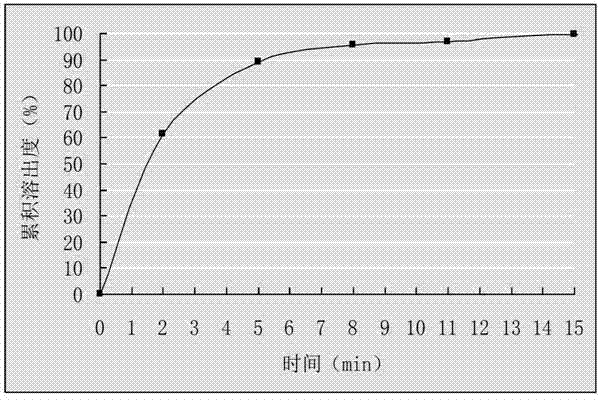
[0030] (2) Mix the drug-loaded pellets obtained in step (1) evenly with talcum powder, and put them into capsule shells to obtain cefuroxime axetil capsules. First get 6 cefuroxime axetil capsules that this example prepares, put into the sedimentation blue respectively, take 0.07mol / L hydrochloric acid solution 900mL as dissolution medium, rotating speed 55r / min, operate according to law, at 0, 2, 5, 8, At 11 and 15 minutes, 5 mL samples were taken for measurement and the cumulative dissolution percentage was calculated. Take the time t as the abscissa, and the average cumulative dissolution percentage is plotted as the ordinate, and the results are sho...
Embodiment 3
[0032]
[0033] Preparation Process:
[0034] (1) Dissolve cefuroxime axetil in 1 liter of acetone, add croscarmellose sodium, stir, grind with a ball mill, control the particle size of the suspension D80<25 microns, and put the formed suspension in the flow Spray and coat the lactose pellets in the fluidized bed to obtain drug-loaded pellets.
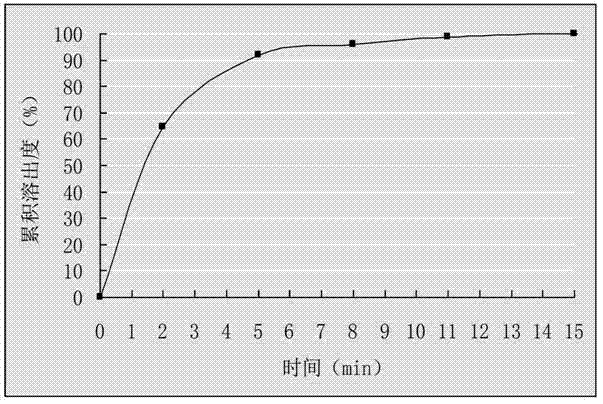
[0035] (2) Mix the drug-loaded pellets obtained in step (1) evenly with talcum powder, and put them into capsule shells to obtain cefuroxime axetil capsules. Get 6 cefuroxime axetil capsules that this example prepares, put into the sedimentation blue respectively, take 0.07mol / L hydrochloric acid solution 900mL as dissolution medium, rotating speed 55r / min, operate according to law, at 0, 2, 5, 8, 11 , 15min, 5mL samples were taken for measurement and the cumulative dissolution percentage was calculated. Take the time t as the abscissa, and the average cumulative dissolution percentage is plotted as the ordinate, and the results a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com