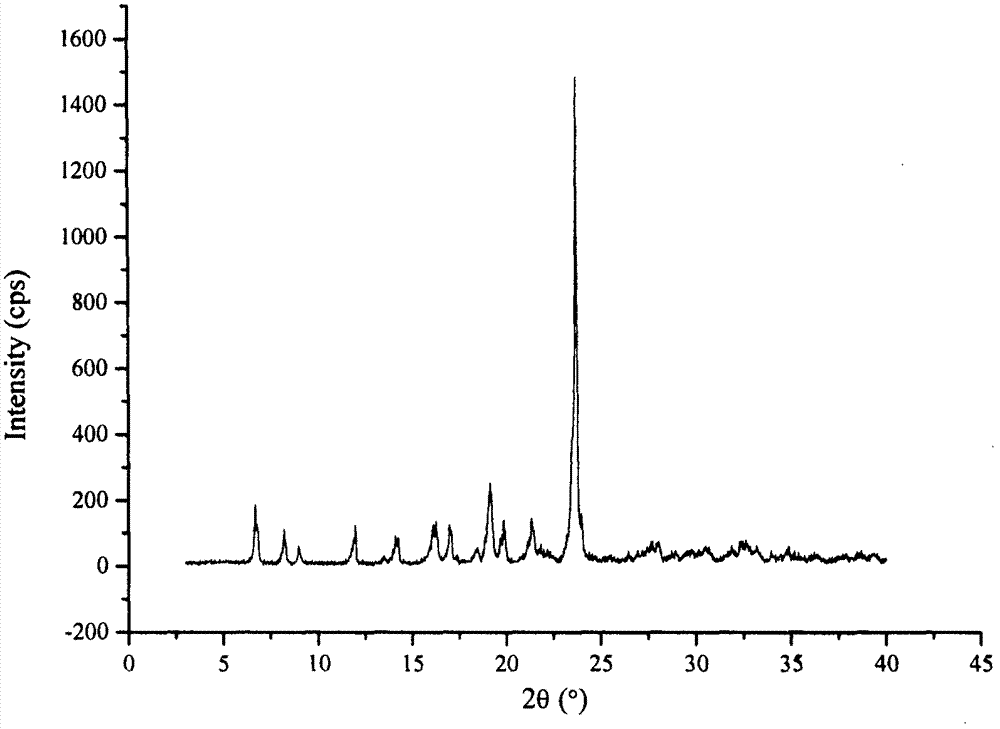
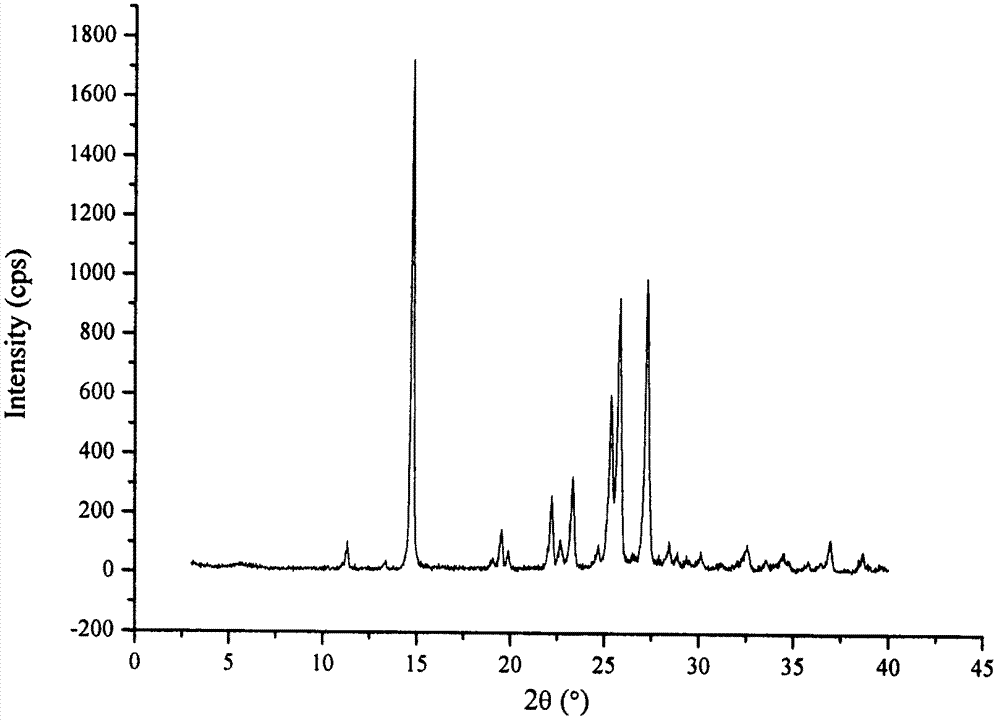
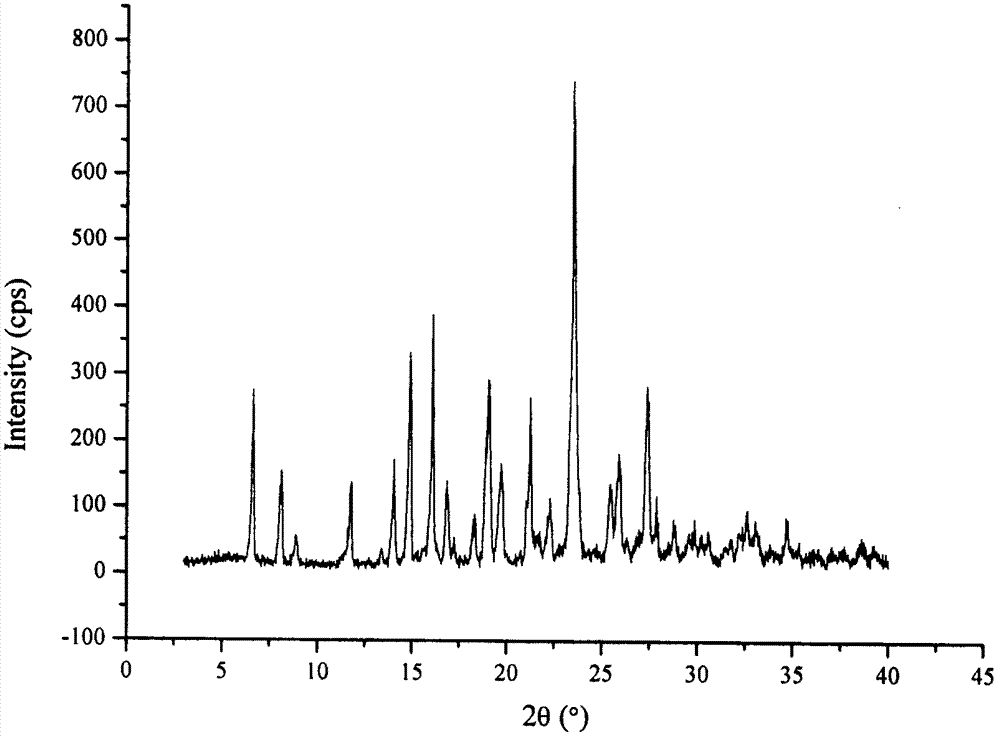
Co-amorphous substance of puerarin nicotinamide
An amorphous, plain nicotinamide technology, applied in the field of medicine, can solve problems such as delayed allergic reactions, frequent, and limited clinical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of puerarin nicotinamide co-amorphous
[0067] Add 2.00g of puerarin and 0.58g of nicotinamide into 50mL of methanol, and ultrasonically dissolve at room temperature to obtain a clear solution. The clear solution is rotatated to evaporate the solvent under reduced pressure at 35°C, and dried in vacuum at 25°C for 12h to obtain 2.34g of white powder.
Embodiment 2
[0068] Example 2: Preparation of puerarin nicotinamide co-amorphous
[0069] Add 2.00g of puerarin and 0.58g of nicotinamide into 50mL of ethanol, and ultrasonically dissolve at room temperature to obtain a clear solution. The clear solution is evaporated under reduced pressure at 50°C and dried in vacuum at 25°C for 12 hours to obtain 2.28g of white powder.
Embodiment 3
[0070] Example 3: Preparation of puerarin nicotinamide co-amorphous
[0071] Add 2.00g of puerarin and 0.58g of nicotinamide into 50mL of methanol-ethanol (50:50, v / v) mixed solvent, and ultrasonically dissolve at room temperature to obtain a clear solution. °C and vacuum dried for 12 hours to obtain 2.32 g of white powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com