Cefuroxime axetil tablets and preparation method thereof
A technology for cefuroxime axetil and furoxamil tablets is applied in the field of cefuroxime axetil tablets and their preparation, which can solve the problems of good fluidity, increased cost of auxiliary materials, complicated processes, etc., and achieves improved drug dissolution rate and improved bioavailability The effect of high degree and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Preparation Process:
[0026] (1) Dissolve cefuroxime axetil in 1 liter of acetone, add crospovidone, stir, grind in a ball mill, control the particle size of the suspension D80<25 microns, and place the formed suspension in a fluidized bed Spray and coat the lactose pellets to obtain drug-loaded pellets.
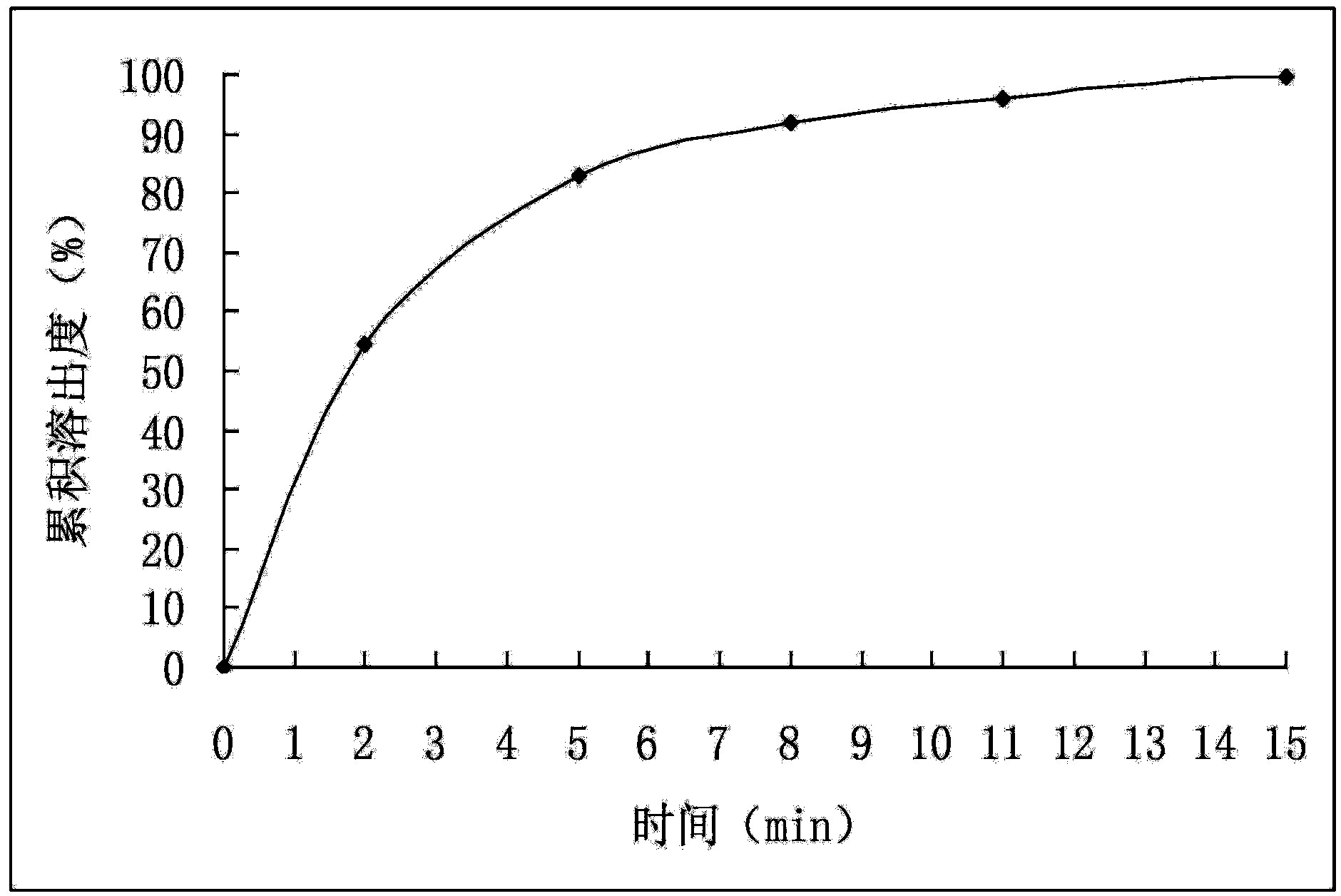
[0027] (2) Mix the drug-loaded pellets obtained in step (1) with mannitol and magnesium stearate evenly, and directly compress into tablets to obtain cefuroxime axetil tablets. The dissolution rate of Cefuroxime Axetil Tablets is detected according to the method under the item of Cefuroxime Axetil Tablets in the "Chinese Pharmacopoeia" 2010 edition, and the cumulative dissolution percentage is calculated, with time t as the abscissa and the average cumulative dissolution percentage as the ordinate, and the results are shown in figure 1 (before accelerated test). The test results showed that the dissolution rate of the cefuroxime axetil tablet prepared i...
Embodiment 2
[0029]
[0030] Preparation Process:
[0031] (1) Dissolve cefuroxime axetil in 1 liter of acetone, add crospovidone, stir, grind in a ball mill, control the particle size of the suspension D80<25 microns, and place the formed suspension in a fluidized bed Spray and coat the lactose pellets to obtain drug-loaded pellets.
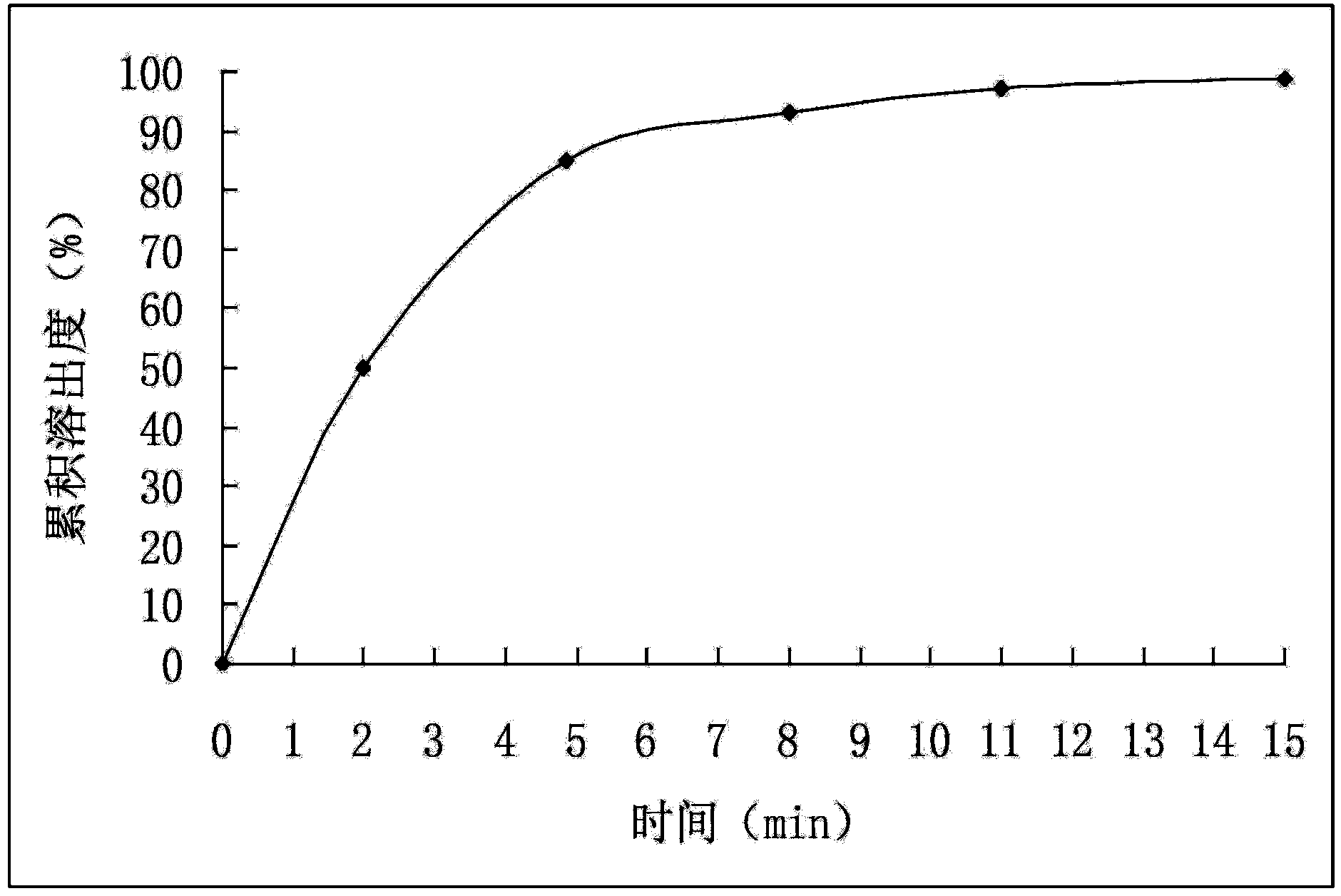
[0032] (2) Mix the drug-loaded pellets obtained in step (1) evenly with pregelatinized starch and magnesium stearate, and directly compress into tablets to obtain cefuroxime axetil tablets. The dissolution rate of Cefuroxime Axetil Tablets is detected according to the method under the item of Cefuroxime Axetil Tablets in the "Chinese Pharmacopoeia" 2010 edition, and the cumulative dissolution percentage is calculated, with time t as the abscissa and the average cumulative dissolution percentage as the ordinate, and the results are shown in figure 2 (before accelerated test). The test results showed that the dissolution rate of the cefuroxime axetil tab...
Embodiment 3
[0034]
[0035] Preparation Process:
[0036] (1) Dissolve cefuroxime axetil in 1 liter of acetone, add croscarmellose sodium, stir, grind with a ball mill, control the particle size of the suspension D80<25 microns, and put the formed suspension in the flow Spray and coat the lactose pellets in the fluidized bed to obtain drug-loaded pellets.
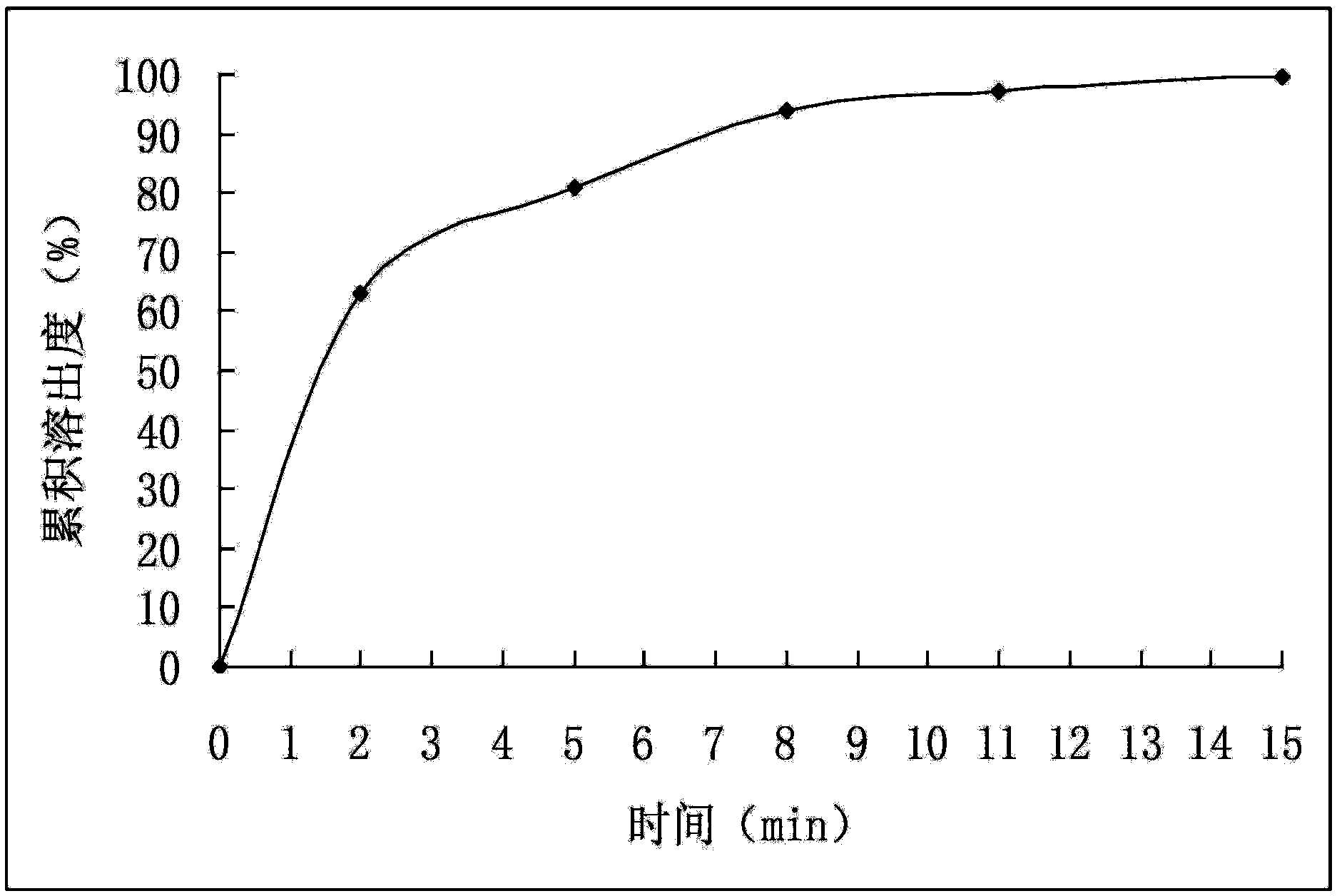
[0037](2) Mix the drug-loaded pellets obtained in step (1) with pregelatinized starch and magnesium stearate evenly, and directly compress into tablets to obtain cefuroxime axetil tablets. The dissolution rate of Cefuroxime Axetil Tablets is detected according to the method under the item of Cefuroxime Axetil Tablets in the "Chinese Pharmacopoeia" 2010 edition, and the cumulative dissolution percentage is calculated, with time t as the abscissa and the average cumulative dissolution percentage as the ordinate, and the results are shown in image 3 (before accelerated test). The test results showed that the dissolution rate of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com