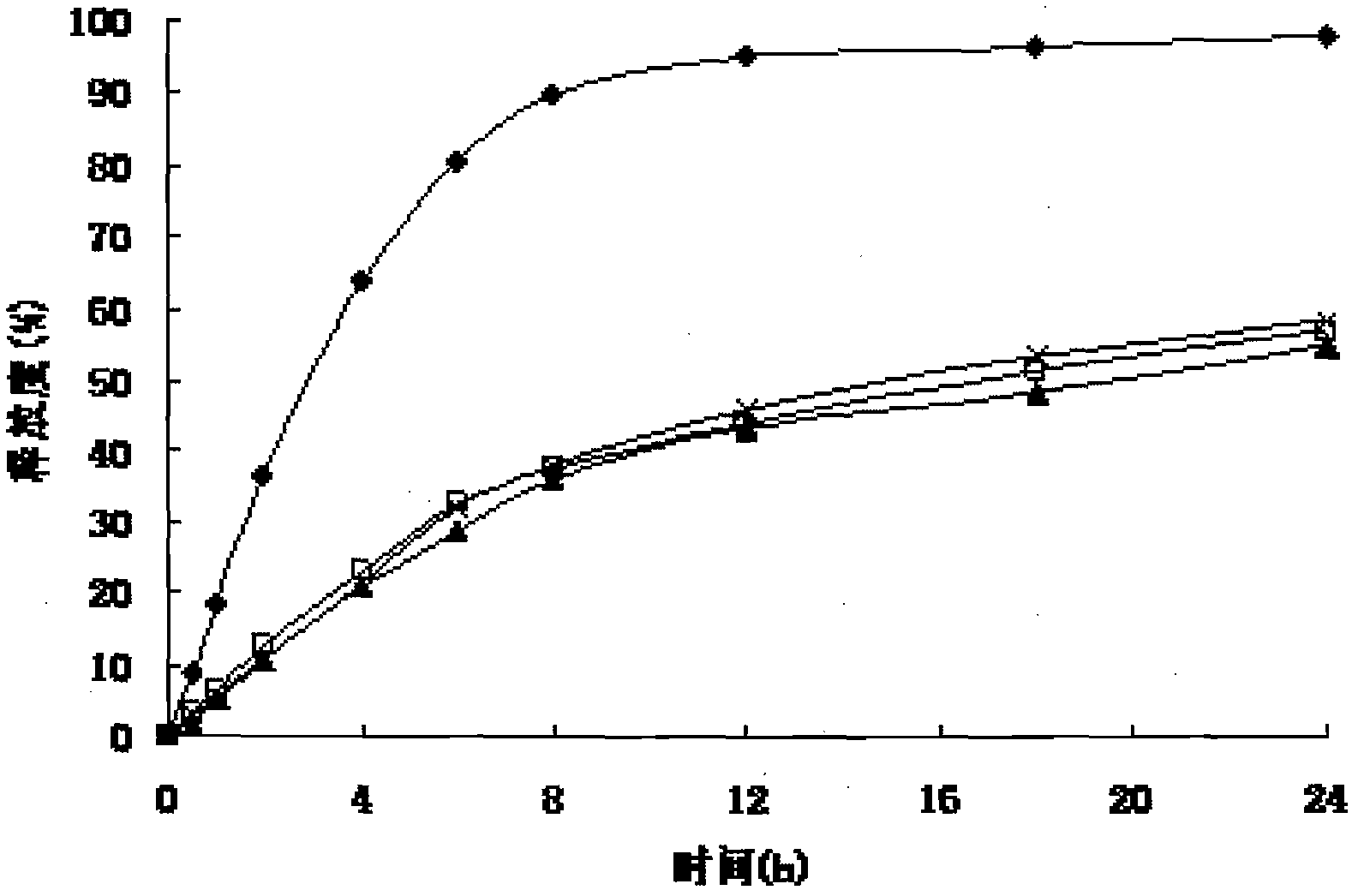
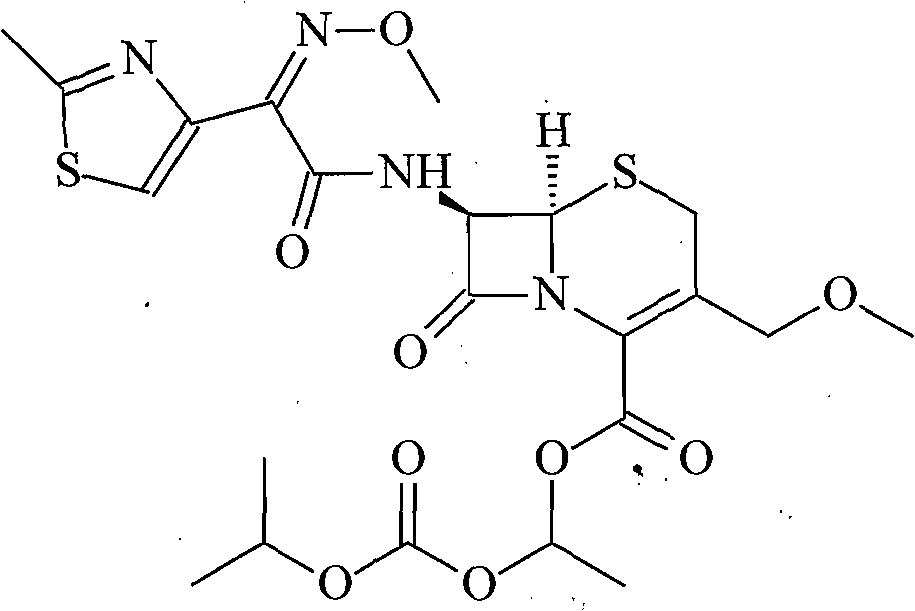
Solid cefpodoxime proxetil liposome preparation
A technology of cefpodoxime axetil and solid preparations, which is applied in the field of medicine to achieve the effects of improving the product quality of preparations, improving the dissolution of preparations, and improving the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] On the other hand, the present invention provides the preparation method of cefpodoxime axetil liposome, the method comprises the following steps:
[0070] (a) dissolving cefpodoxime axetil, β-sitosterol, stearylamide, cholesterol, and Tween 80 in an organic solvent, stirring to dissolve it;
[0071] (b) Place the above solution in an eggplant-shaped bottle, remove the organic solvent under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0072] (c) Add buffer solution to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0073] (d) The above solution is filtered with a 0.45 microporous membrane, the filtrate is placed in a -20°C refrigerator and frozen overnight, then taken out and thawed, repeated freezing and thawing three times, and spray-dried to obtain cefpodoxime axetil liposome powder.
[0074] In a preferred embodiment of the me...
Embodiment 1
[0117] Embodiment 1 cefpodoxime axetil liposome sheet
[0118] The raw materials used are as follows:
[0119]
[0120] Adopt following production process to prepare cefpodoxime axetil liposome tablet:
[0121] (1) Accurately weigh 50g cefpodoxime axetil, 160g β-sitosterol, 40g stearamide, 100g cholesterol, 50g Tween 80, dissolve in 1200ml volume ratio of 2:1 dichloromethane and isopropanol and mix In the solvent, stir to dissolve it;
[0122] (2) Place the above solution in an eggplant-shaped bottle, remove methylene chloride and isopropanol under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0123] (3) Add 1200ml of phosphate buffer solution with a pH value of 6.8 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0124] (4) above-mentioned solution is filtered with 0.45 microporous membrane, and filtrate is placed in-20 ℃ of ref...
Embodiment 2
[0128] Embodiment 2 cefpodoxime axetil liposome capsule
[0129]
[0130] Adopt following production process to prepare cefpodoxime axetil liposome capsule:
[0131] (1) Accurately weigh 100g cefpodoxime axetil, 100g β-sitosterol, 25g stearamide, 150g cholesterol, 80g Tween 80, dissolve in 1000ml volume ratio of 2:1 dichloromethane and isopropanol and mix In the solvent, stir to dissolve it;
[0132] (2) Place the above solution in an eggplant-shaped bottle, remove methylene chloride and isopropanol under reduced pressure in a 45°C water bath, and form a uniform transparent film on the wall of the bottle;
[0133] (3) Add 1000 ml of phosphate buffer solution with a pH value of 6.8 to the eggplant-shaped bottle, and continue to rotate in a 45°C water bath under normal pressure to swell and hydrate the film;
[0134] (4) above-mentioned solution is filtered with 0.45 microporous membrane, and filtrate is placed in-20 ℃ of refrigerators and freezes overnight, then takes ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com