Glipizide tablet and its preparation method and application
A technology of glipizide tablets and glipizide, which is applied in the direction of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., to achieve the effects of improving bioavailability, increasing drug release, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
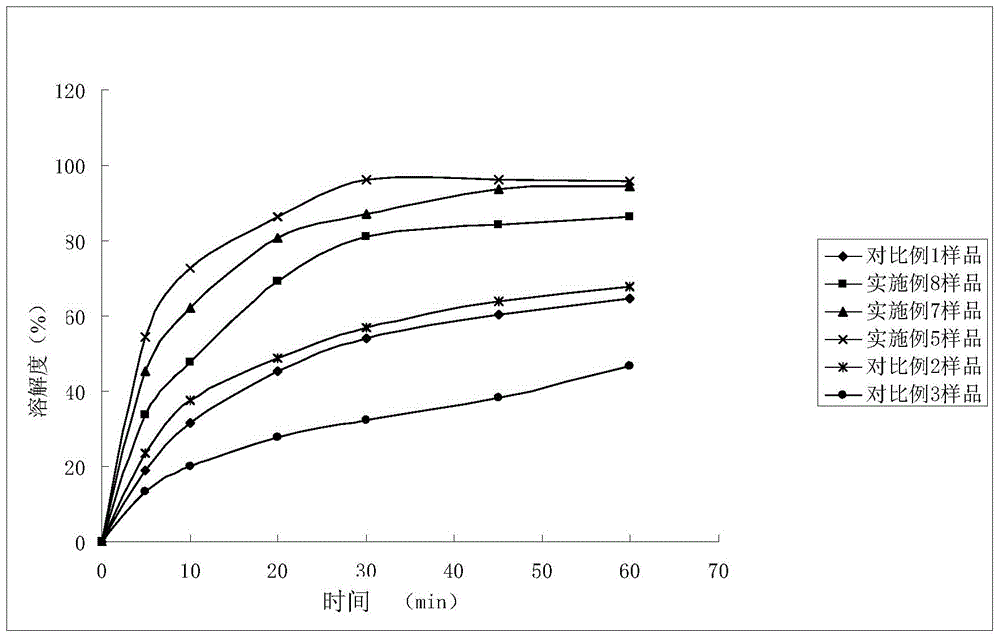
Image
Examples
Embodiment 1
[0027] prescription
[0028]
[0029] Preparation Process
[0030] (1) take starch (adhesive) and be mixed with purified water into 8wt% starch slurry, as adhesive;
[0031] (2) Glipizide is micronized to obtain the glipizide whose particle size D50 is 100 μm, and the glipizide that has been sieved is weighed according to the prescription amount, mixed with microcrystalline cellulose, starch, poloxa The sodium carboxymethyl starch of 4 / 5 in the total amount and the prescription quantity is mixed by the method of equal increment;
[0032] (3) Add an appropriate amount of binder to make soft materials, pass through a 20-mesh sieve to granulate, dry the wet granules at 40°C, and pass through a 20-mesh sieve for granulation after drying for 1 hour;
[0033] (4) the magnesium stearate and the remaining sodium carboxymethyl starch are mixed with the dry granule after the granulation and the prescription amount;
[0034] (5) After mixing evenly, use a Φ6.5mm shallow concave pun...
Embodiment 2
[0036] prescription
[0037]
[0038] Preparation Process
[0039] (1) Take by weighing the prescription amount of povidone K30, join concentration and be in the 50wt% ethanol solution, be mixed with 5wt% povidone K30-ethanol solution, use this as adhesive;
[0040] (2) Glipizide is micronized to obtain Glipizide whose particle size D50 is 75 μm, and the micronized Glipizide is weighed according to the prescription amount, mixed with microcrystalline cellulose, starch, poloxamer And the crospovidone of 4 / 5 in the prescription quantity is mixed by the equal amount increasing method;
[0041](3) Add an appropriate amount of binder to make soft materials, pass through a 20-mesh sieve to granulate, dry the wet granules at 40°C, and pass through a 20-mesh sieve for granulation after drying for 1 hour;
[0042] (4) mix the dry granule after granulation with the magnesium stearate of the prescription amount and the remaining crospovidone;
[0043] (5) After mixing evenly, use a...
Embodiment 3
[0045] prescription
[0046]
[0047]
[0048] Preparation Process
[0049] (1) Take by weighing the prescription amount of povidone K30, join concentration and be in the 50wt% ethanol solution, be mixed with 5wt% povidone K30-ethanol solution, use this as adhesive;
[0050] (2) Glipizide is micronized to obtain the glipizide whose particle size D50 is 50 μm, and the micronized glipizide is weighed according to the prescription quantity, mixed with microcrystalline cellulose, starch, SDS and prescription quantity The sodium carboxymethyl starch of 4 / 5 is mixed according to the method of equal increment;
[0051] (3) Add an appropriate amount of binder to make soft materials, pass through a 20-mesh sieve to granulate, dry the wet granules at 40°C, and pass through a 20-mesh sieve for granulation after drying for 1 hour;
[0052] (4) the magnesium stearate and the remaining sodium carboxymethyl starch are mixed with the dry granule after the granulation and the prescript...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com