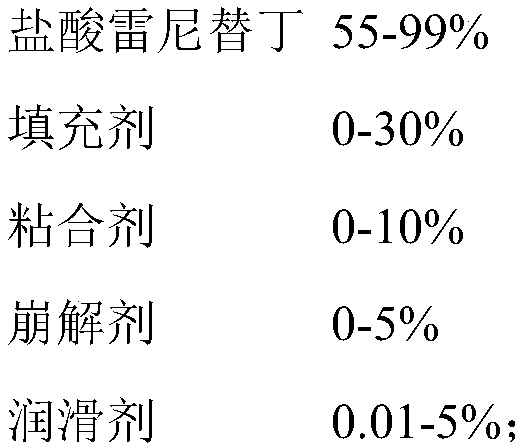
Industrial preparation method of ranitidine hydrochloride capsule
A technology of ranitidine hydrochloride and capsules, which is applied in the field of industrial preparation of ranitidine hydrochloride capsules, can solve the problems of unfavorable product stability, instability of ranitidine hydrochloride, long process cycle, etc., and achieve good bioavailability , Good drug dissolution rate, no discoloration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
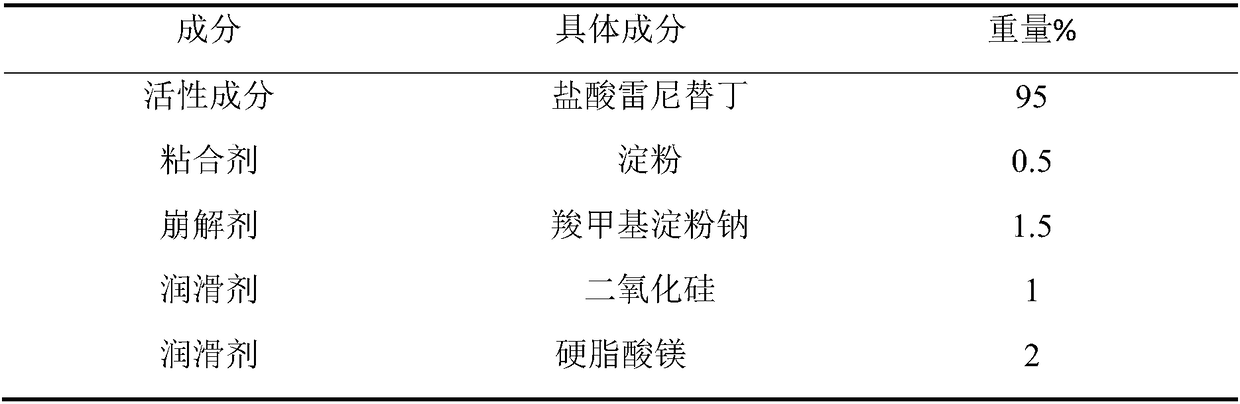
[0066] The preparation of embodiment 1 ranitidine hydrochloride capsules of the present invention
[0067]
[0068] Preparation Process:
[0069] The ranitidine hydrochloride crude drug that takes prescription dosage is placed in fluidized bed, and the starch slurry solution of 3% concentration is sprayed in the fluidized bed with atomization form, atomization pressure 0.3MPa, spray speed 3g / kg / min, control the temperature of the material at 25-30°C, and granulate ranitidine hydrochloride; continue to dry the material with a fluidized bed until the moisture is less than 3%; pass the granule material through a 40-mesh sieve for granulation, and the particle size is uniform , white to light yellow. After adding supplementary materials such as carboxymethyl starch sodium, silicon dioxide and magnesium stearate in prescription dosage and mixing for 10 minutes, the ranitidine hydrochloride capsule product whose capsule content was 175 mg was prepared by filling.
Embodiment 2
[0070] The preparation of embodiment 2 ranitidine hydrochloride capsules of the present invention
[0071]
[0072] Preparation Process:
[0073] Weigh the ranitidine hydrochloride and starch of the prescribed amount and mix them in the fluidized bed and be in a fluidized state, spray water into it, the atomization pressure is 0.2MPa, the spray speed is 4g / kg / min, preheat and control The mixed material is granulated at a temperature of 30-35°C. After the mixed material is in good condition, the prepared granular material is dried until the moisture content is not higher than 3%; the granular material is passed through a 40-mesh screen for granulation, and the particle size is uniform. It is white to light yellow. After adding auxiliary materials such as croscarmellose sodium, talcum powder and magnesium stearate in prescription dosage and mixing for 15 minutes, the ranitidine hydrochloride capsule product whose capsule content was 210 mg was prepared by filling.
Embodiment 3
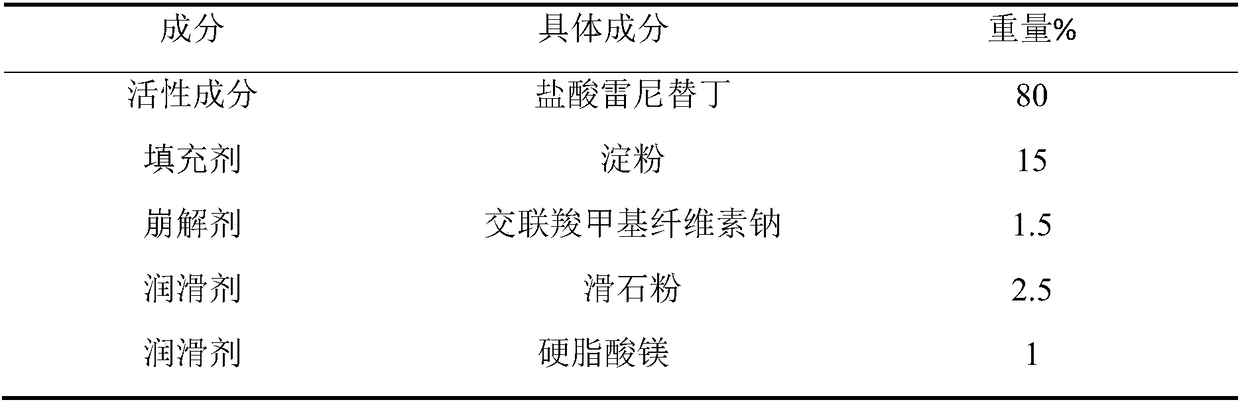
[0074] The preparation of embodiment 3 ranitidine hydrochloride capsules of the present invention
[0075]
[0076] Preparation Process:
[0077] The ranitidine hydrochloride crude drug and lactose that take prescription quantity are mixed in the fluidized bed, the povidone aqueous solution of 8% concentration is atomized and sprayed in the fluidized bed, atomization pressure 0.5MPa, spray speed 0.2g / kg / min, preheat and control the temperature of the material at 35-40°C before granulating, continue to dry the granular material in the fluidized bed until the moisture content is not higher than 3%; pass the granular material through a 40-mesh screen for granulation, The particle size is uniform, white to light yellow in color. After adding excipients such as low-substituted hydroxypropyl cellulose, silicon dioxide and magnesium stearate in the prescribed amount and mixing for 30 minutes, the ranitidine hydrochloride capsule product with a capsule content of 280 mg was prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com