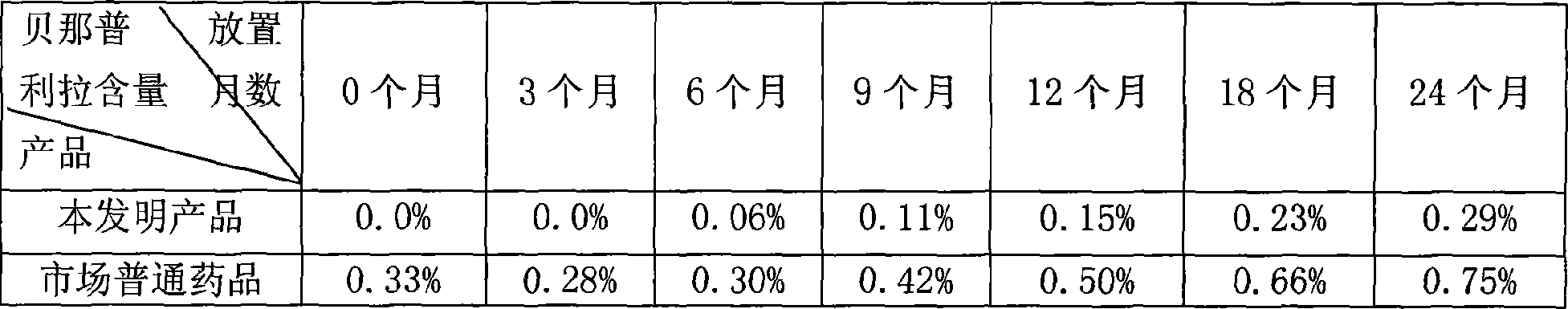
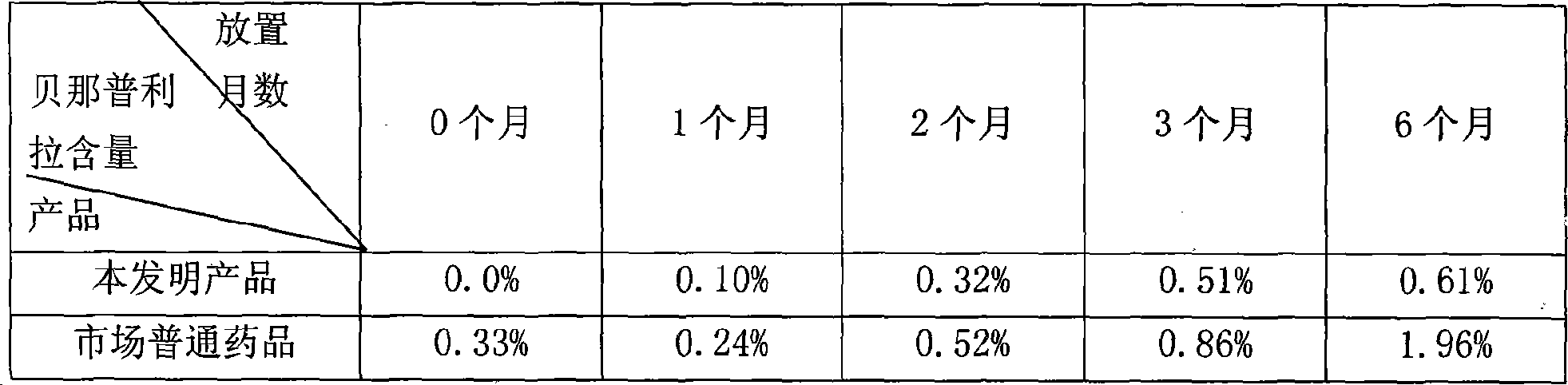
Benazepril pharmaceutical compsn. and process for its prepn.
A technology for compositions and medicines, applied in the directions of medicine combinations, medicine formulations, active ingredients of heterocyclic compounds, etc., can solve the problems of serious pollution, high cost, complicated preparation process, etc., and achieves a high degree of automation, high production efficiency, and process equipment. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Benazepril hydrochloride 15.0g, pregelatinized starch 45.0g, mannitol 180.0g, polyethylene glycol (40) monostearate 3.5g, sucrose higher fatty acid ester 4.0g, glycerin palmitic acid hard After mixing 2.7g of fatty acid esters with the method of equal increase, mix and pulverize in a ball mill, after pulverizing for 30 minutes, raise the temperature of the material to 40°C to 50°C, grind and pulverize for 30 minutes, take out the material and let it cool, Use a fast granulator with a 1.0mm sieve to granulate and pulverize, and sieve fine particles above 60 mesh.
[0039] Mix the obtained granules with 13.5 g of disintegrating agent sodium carboxymethyl starch and 2.7 g of lubricant micropowder silica gel, and then press into tablets with a tablet machine, and the hardness is controlled at 2.0 kg / cm 2 up to 5.0kg / cm 2 , made into tablets with a content of 5 mg and a tablet weight of 0.09 g.
Embodiment 2
[0041] Benazepril hydrochloride 15.0g, pregelatinized starch 70.0g, mannitol 180.0g, polyethylene glycol (40) monostearate 8.0g, sucrose higher fatty acid ester 2.4g, glycerin palmitic acid hard After mixing 3.5 g of fatty acid esters by the method of increasing in equal amounts, mix and pulverize in a ball mill, and after pulverizing for 30 minutes, raise the temperature of the material to 40°C to 50°C, grind and pulverize for another 30 minutes, take out the material and let it cool, Use a fast granulator with a 1.0mm sieve to granulate and pulverize, and sieve fine particles above 60 mesh.
[0042] Mix the obtained granules with 14.5 g of disintegrant sodium carboxymethyl starch and 3.5 g of lubricant micropowder silica gel, and then press into tablets with a tablet machine, and the hardness is controlled at 2.0 kg / cm 2 up to 5.0kg / cm 2 , made into tablets with a content of 10 mg and a tablet weight of 0.18 g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com