Memantine hydrochloride sustained-release pellet and preparation method thereof
A technology of memantine hydrochloride and sustained-release pellets, applied in microcapsules, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as different dosage forms, increased solvent costs, and poor water solubility of ethyl cellulose, and achieve The production operation process is safe, the production energy consumption is avoided, and the stability is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
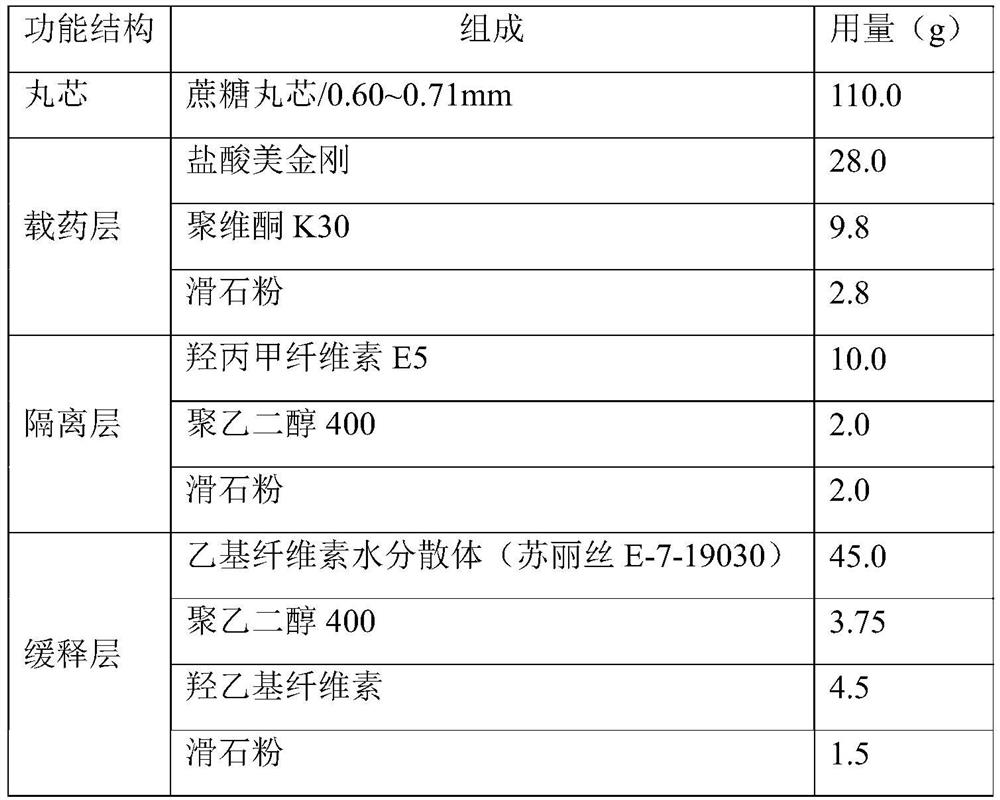
[0036] prescription:
[0037]
[0038] Preparation Process:
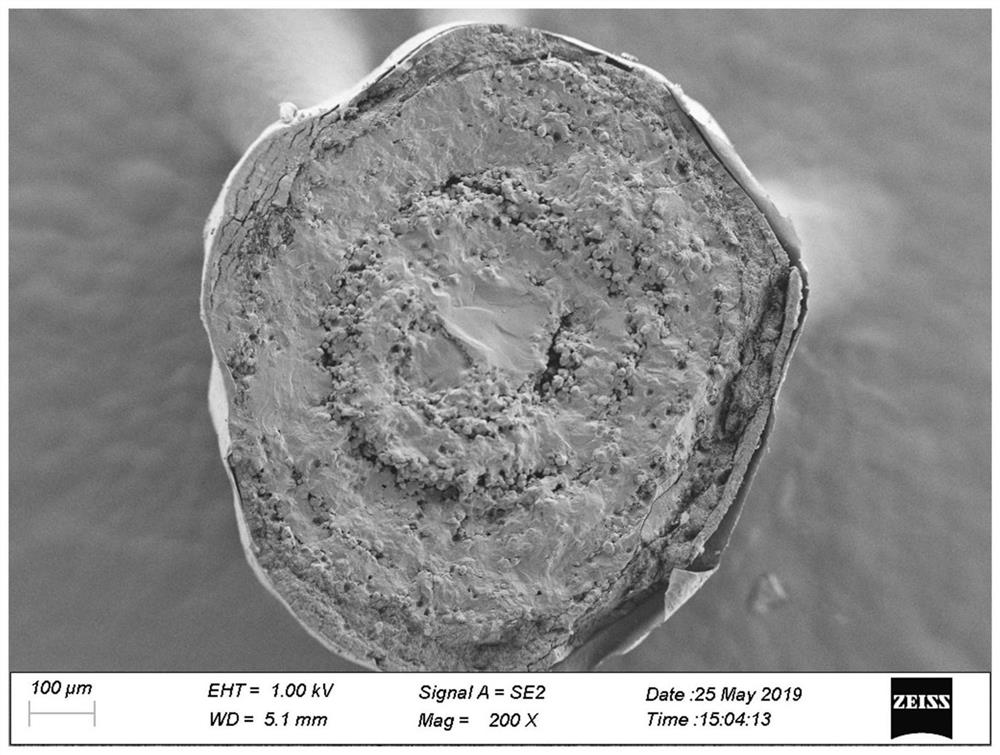
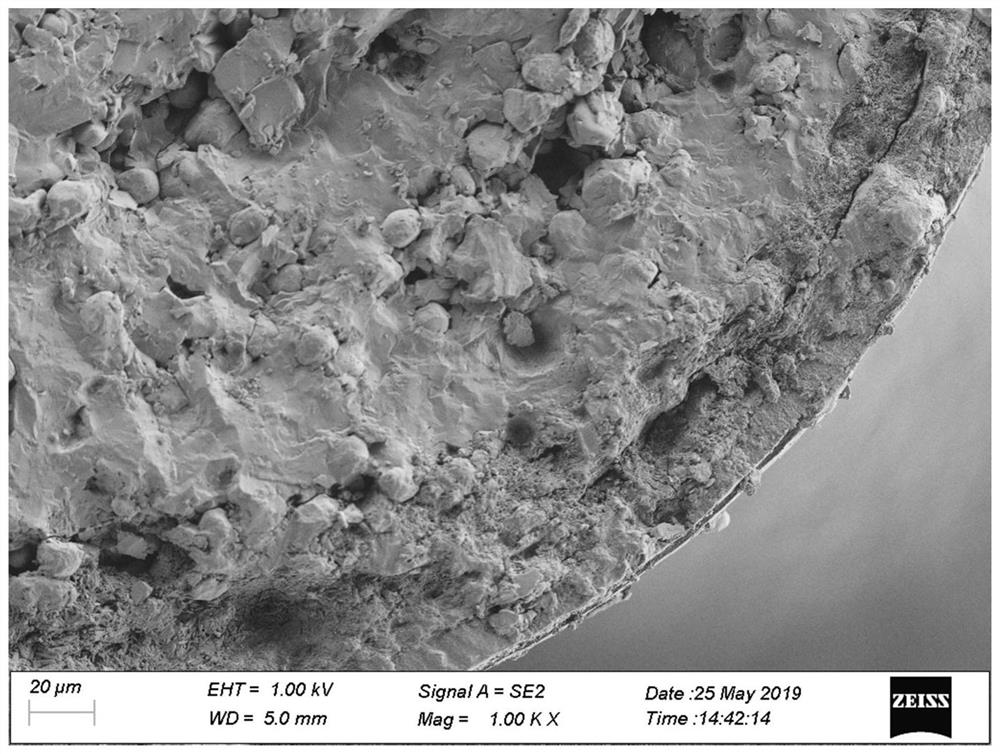
[0039] (1) drug-loading layer coating: Memantine hydrochloride raw material is pulverized by a jet mill to obtain memantine hydrochloride micropowder, particle diameter D 90 Controlled within the range of 15 μm; Memantine hydrochloride micropowder, povidone K30 and talc were dispersed in an appropriate amount of purified water to prepare a drug-loading layer coating liquid; The coating liquid of the drug-loading layer is wrapped on the surface of the sucrose pellet core by spraying method. During the coating process, the temperature of the material is controlled within the range of 25°C to 35°C. When the temperature of the material is below 35°C, the drug-loaded layer pellets are obtained.
[0040] (2) Coating of isolation layer: add hypromellose E5 to purified water at 60°C to 80°C, stir and disperse evenly, cool to room temperature, add polyethylene glycol 400 and talc, stir evenly, and prepare an isolation l...
Embodiment 2
[0044] prescription:
[0045]
[0046] Preparation Process:
[0047] (1) drug-loading layer coating: Memantine hydrochloride raw material is pulverized by a jet mill to obtain memantine hydrochloride micropowder, particle diameter D 90 Controlled within the range of 15 μm; Memantine hydrochloride micropowder, povidone K30 and talc were dispersed in an appropriate amount of purified water to prepare a drug-loading layer coating liquid; The coating liquid of the drug-loading layer is wrapped on the surface of the sucrose pellet core by spraying method. During the coating process, the temperature of the material is controlled within the range of 25°C to 35°C. When the temperature of the material is below 35°C, the drug-loaded layer pellets are obtained.
[0048] (2) Coating of isolation layer: add hypromellose E5 to purified water at 60°C to 80°C, stir and disperse evenly, cool to room temperature, add polyethylene glycol 400 and talc, stir evenly, and prepare an isolation l...
Embodiment 3
[0052] prescription:
[0053]
[0054] Preparation Process:
[0055] (1) drug-loading layer coating: Memantine hydrochloride raw material is pulverized by a jet mill to obtain memantine hydrochloride micropowder, particle diameter D 90 Controlled within the range of 15 μm; Memantine hydrochloride micropowder, povidone K30 and talc were dispersed in an appropriate amount of purified water to prepare a drug-loading layer coating liquid; The coating liquid of the drug-loading layer is wrapped on the surface of the sucrose pellet core by spraying method. During the coating process, the temperature of the material is controlled within the range of 25°C to 35°C. When the temperature of the material is below 35°C, the drug-loaded layer pellets are obtained.
[0056] (2) Coating of isolation layer: add hypromellose E5 to purified water at 60°C to 80°C, stir and disperse evenly, cool to room temperature, add polyethylene glycol 400 and talc, stir evenly, and prepare an isolation l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com