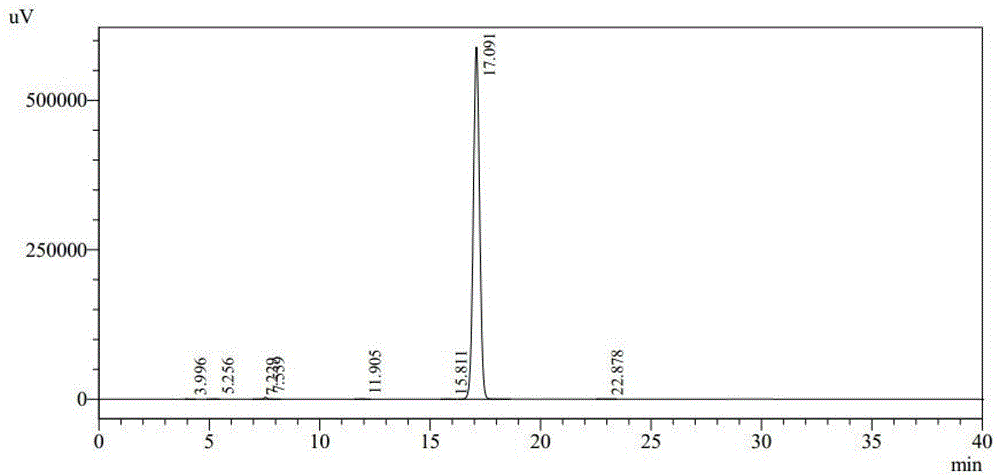
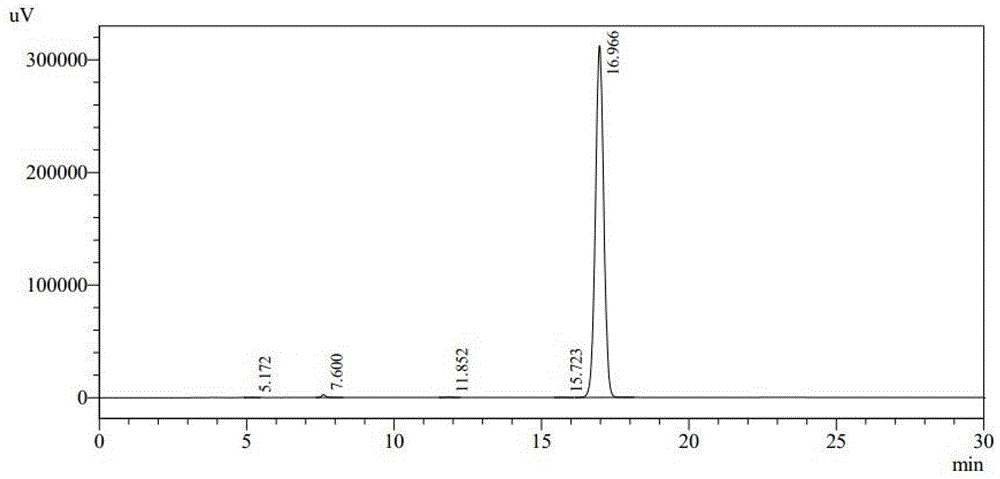
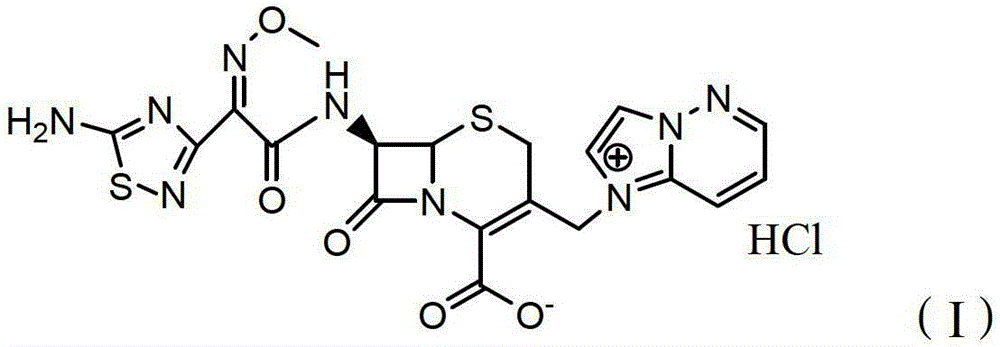
Preparation method of freeze-dried preparation of cefozopran hydrochloride
A technology of cefazolam hydrochloride and freeze-dried preparations is applied in the field of preparation of freeze-dried preparations of cefazolam hydrochloride for injection, which can solve the problem that it is difficult to remove the solvent to a satisfactory level, the preparation method is complicated, and it is difficult to prepare qualified and high-quality preparations. Cefazolam hydrochloride freeze-dried preparation and other problems, to achieve the effect of excellent stability, purity and appearance, less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] (1) Dissolve 206g of anhydrous sodium carbonate and 120g of sodium chloride in 5L of water for injection at room temperature, and use CO 2 Adjust the pH value to about 9 to obtain the excipient solution;
[0072] (2) Add 5L of water for injection and 3L of absolute ethanol into a 20L reaction kettle, preheat to 50°C, add 1098.5g of cefazolam hydrochloride with 2.3% water and 0.3% ethanol content (ceftozolam C 19 h 17 N 9 o 5 S 2 Calculated as 1000g), stirred and dissolved to obtain cefzolam hydrochloride water-ethanol solution;
[0073] (3) Add the excipient solution prepared in step (1) dropwise to the cefzolam hydrochloride water-ethanol solution prepared in step (2), while using CO 2 Control the pH value to about 7.5, drop the temperature naturally after dropping to obtain a mixed solution, and control the temperature of the mixed solution to be 20°C to 25°C;
[0074] (4) Add 50 g of activated carbon to the mixed solution obtained in step (3) and stir for 20 mi...
Embodiment 2
[0097] (1) Dissolve 206g of anhydrous sodium carbonate and 120g of sodium chloride in 6L of water for injection at 35°C to 40°C, and use CO 2 Adjust the pH value to about 10 to obtain the excipient solution;
[0098](2) Add 6L of water for injection and 2L of anhydrous methanol into a 20L reaction bottle, preheat to 37°C, add 1095.1g of cefazolam hydrochloride with 2.1% water and 0.2% methanol content (ceftozolam C 19 h 17 N 9 o 5 S 2 Calculated as 1000g), stirred and dissolved to obtain cefzolam hydrochloride water-methanol solution;
[0099] (3) Add the excipient solution prepared in step (1) dropwise to the cefzolam hydrochloride water-methanol solution prepared in step (2), while using CO 2 Control the pH value to about 8.0 to obtain a mixed solution, and control the temperature of the mixed solution to be 25° C. to 30° C.
[0100] (4) Add 30 g of activated carbon to the mixed solution obtained in step (3) and stir for 30 minutes while maintaining CO 2 , control the...
Embodiment 3
[0124] (1) Dissolve 206g of anhydrous sodium carbonate and 120g of sodium chloride in 3.5L of water for injection at 20°C to 25°C, and use CO 2 Adjust the pH value to 8 to obtain the excipient solution;
[0125] (2) Add 3.5L of water for injection and 2.5L of absolute ethanol into a 20L reactor, preheat to 52°C, add 1183.1g of cefozopran hydrochloride ethanol solvate with 3.0% water and 7.5% ethanol C 19 h 17 N 9 o 5 S 2 Calculated as 1000g), stirred and dissolved to obtain cefzolam hydrochloride water-ethanol solution;
[0126] (3) Add the excipient solution prepared in step (1) dropwise to the cefzolam hydrochloride water-ethanol solution prepared in step (2), while using CO 2 The pH value is controlled to be 7 to obtain a mixed solution, and the temperature of the mixed solution is controlled to be 30° C. to 40° C.
[0127] (4) Add 30 g of needle activated carbon to the mixed solution obtained in step (3) and stir while maintaining CO 2 , the temperature of the mixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com