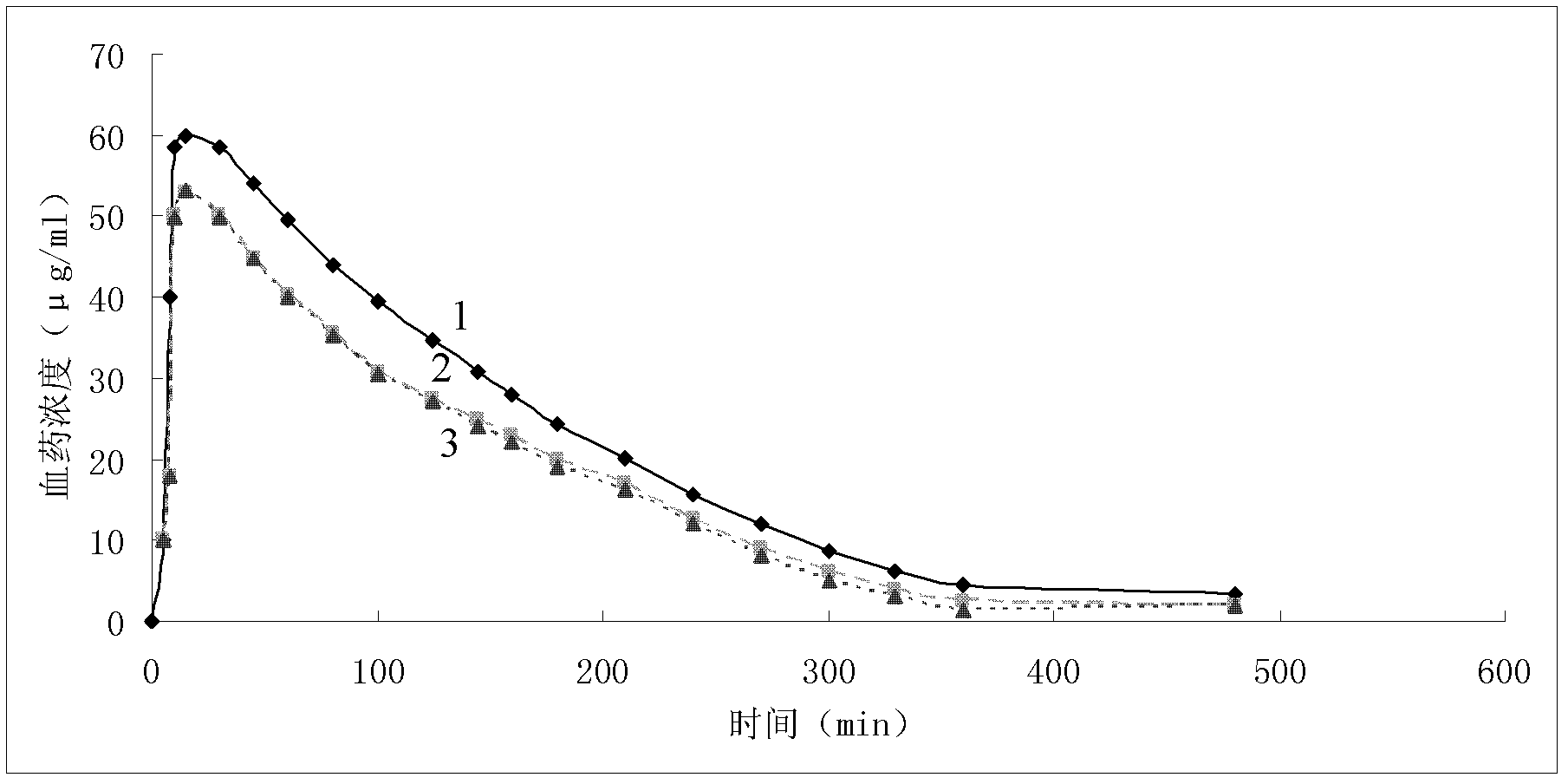
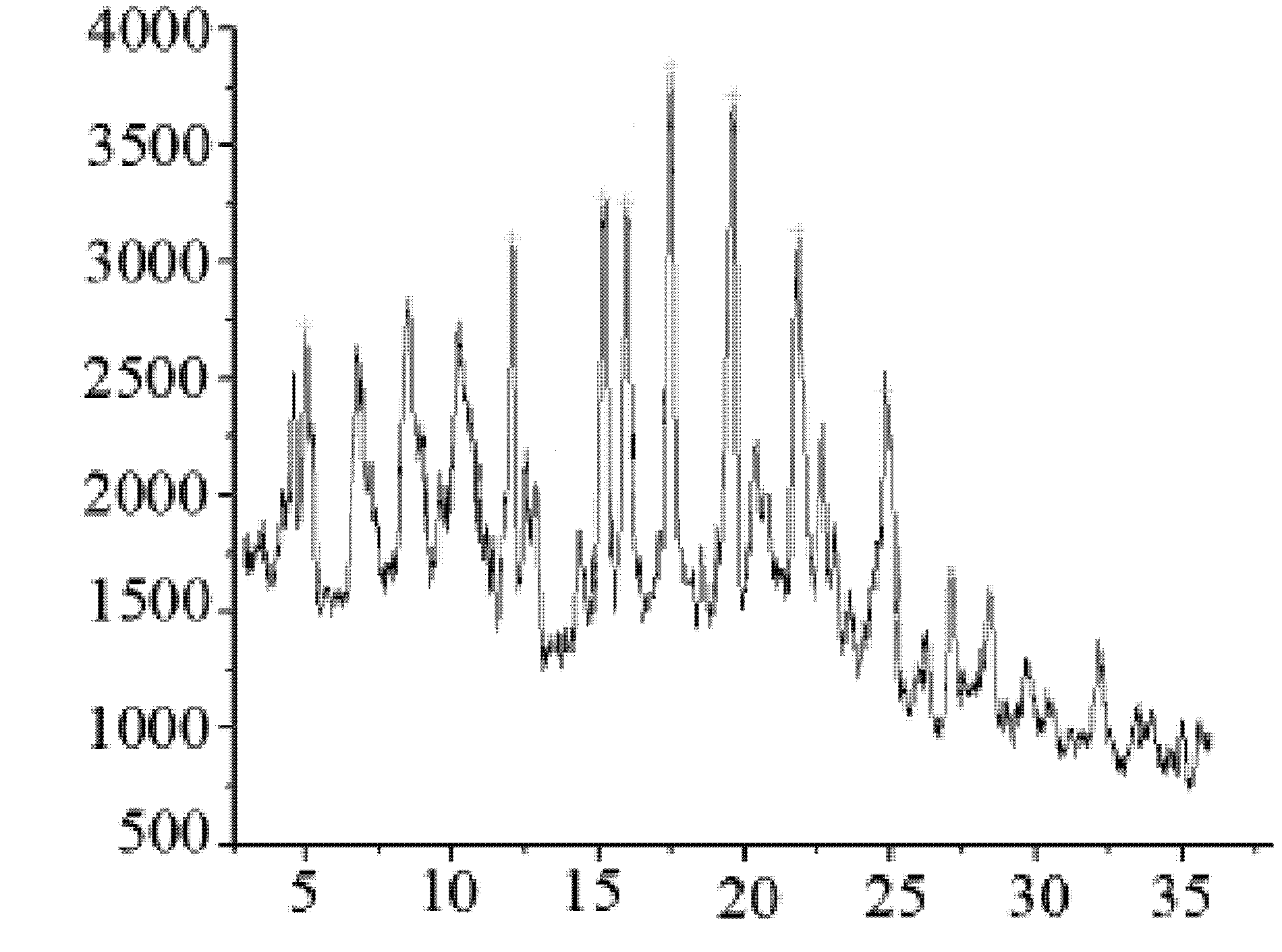
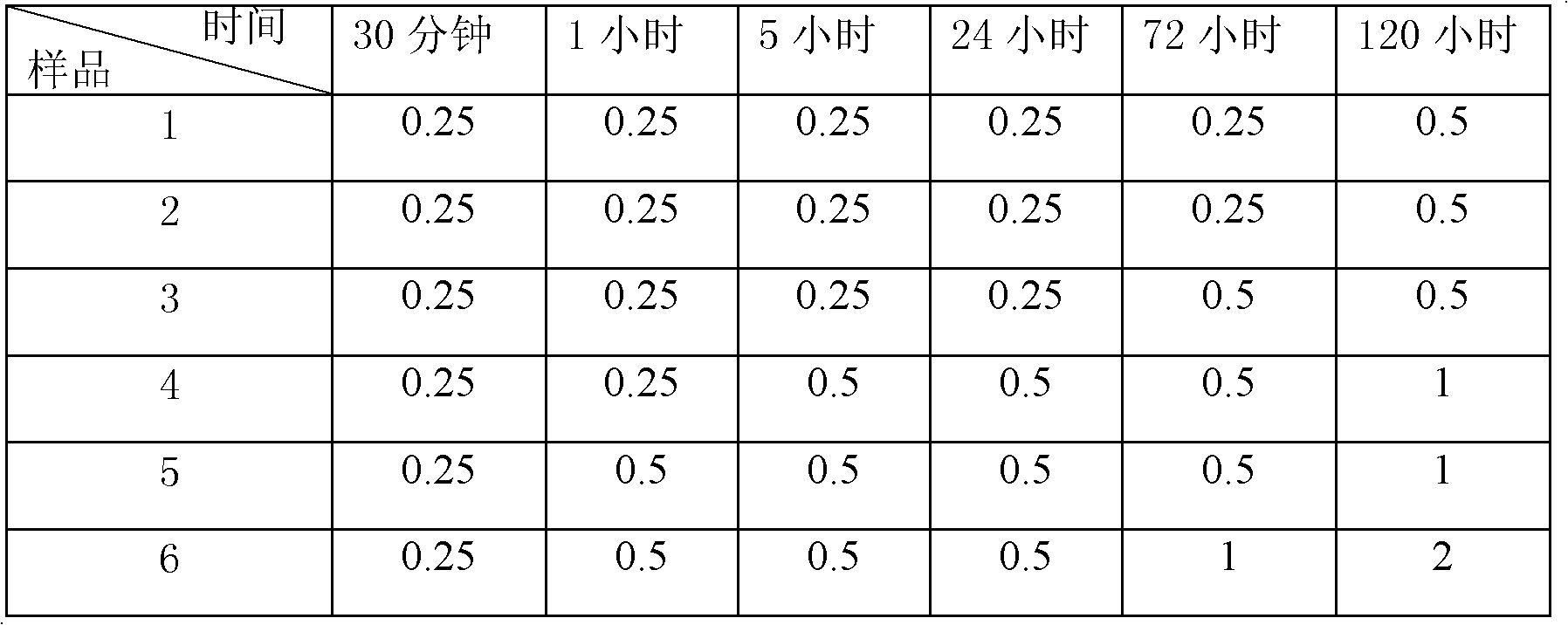
A kind of cefminox sodium crystalline compound and its composition powder injection
A technology of cefminox sodium and compound, applied in the field of medicine, can solve the problems of high price and the like, and achieve the effects of improving bioavailability, improving drug safety, and good dissolution stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of cefminox sodium crystalline compound: get cefminox sodium crude product 1kg, add volume and be that the volume ratio of 8 times of cefminox sodium crude product weight is the water of 6.4: 3.6: methanol mixed solution, be heated to reflux; Cefminox sodium After the crude product dissolves clear, add volume and be the acetone of 2.2 times of cefminox sodium crude product weight, stop heating, under stirring, drip the acetone that volume is 1.2 times of cefminox sodium crude product weight: ethanol solution, described stirring is 27rmp, described Dropping is to control the dropping time for 7 minutes and drop at a constant speed; after dropping, stir and cool down. The stirring and cooling is to cool down to 52°C for 10 minutes while stirring at a rotating speed of 22rmp, and then cool down to 23°C for 20min while stirring at a rotating speed of 7rmp, and let it stand for 19 hours. Filter, wash twice with 8:2 methanol:ethanol solution, 0.5 times each tim...
Embodiment 2
[0050] The preparation of cefminox sodium crystalline compound: get cefminox sodium crude product 2kg, add volume and be that the volume ratio of 8 times of cefminox sodium crude product weight is the water of 6.4: 3.6: methanol mixed solution, be heated to reflux; Cefminox sodium After the crude product dissolves clear, add volume and be the acetone of 2.2 times of cefminox sodium crude product weight, stop heating, add dropwise volume under stirring and be the acetone of 1.2 times of cefminox sodium crude product weight: ethanol solution, described stirring is 25rmp, described Dropping is to control the dropping time for 7 minutes and drop at a constant speed; after dropping, stir and cool down. The stirring and cooling is to cool down to 51°C for 10 minutes under stirring at a rotating speed of 20rmp, and then cool down to 23°C for 20min under stirring at a rotating speed of 7rmp, and stand for 19 hours. Filter, wash twice with 8:2 methanol:ethanol solution, 0.5 times each t...
Embodiment 3
[0053] The preparation of cefminox sodium crystalline compound: get cefminox sodium crude product 10kg, add volume and be that the volume ratio of 8 times of cefminox sodium crude product weight is the water of 6.4: 3.6: methanol mixed solution, be heated to reflux; Cefminox sodium After the crude product dissolves clear, add volume and be the acetone of 2.0 times of cefminox sodium crude product weight, stop heating, add dropwise volume under stirring and be the acetone of 1.0 times of cefminox sodium crude product weight: ethanol solution, described stirring is 24rmp, described Dropping is to control the dropping time for 6 minutes and drop at a constant speed; after dropping, stir and cool down. The stirring and cooling is to cool down to 53°C for 10 minutes under stirring at a rotating speed of 22rmp, and then cool down to 24°C for 20min under stirring at a rotating speed of 8rmp, and stand for 20 hours. Filter, wash twice with 8:2 methanol:ethanol solution, 0.4 times each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com