Doxofylline crystalline compound and lyophilized powder thereof
A technology of doxofylline and freeze-dried powder injection, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of doxofylline crystalline compound
[0035] (1) Take 10 g of commercially available doxofylline crude product and fully dissolve it in 45 ml of a mixed solution of water / n-butanol / acetonitrile with a volume ratio of 4:3:1.5, heat to reflux, stir and dissolve to obtain a doxofylline solution ;
[0036] (2) Add dropwise 9ml of ether / methanol solution with a volume ratio of 4:1 to the doxofylline solution under reflux and stirring, and finish dropping in 8-10 minutes;
[0037] (3) Stop heating, stir at a rotating speed of 50rmp and cool down to 10°C, wherein the cooling rate is 8°C / 5min, stand still for 18h to crystallize, filter, wash the filter cake with 5ml of ether, and vacuum-dry the crystalline compound of doxofylline, Yield 88.5%, HPLC 99.54%, mp 178~181℃.
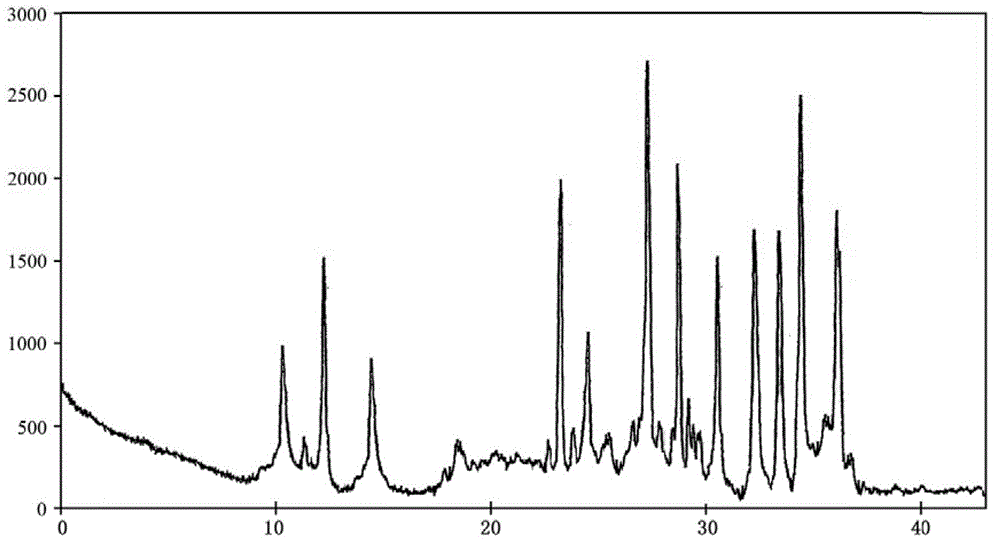
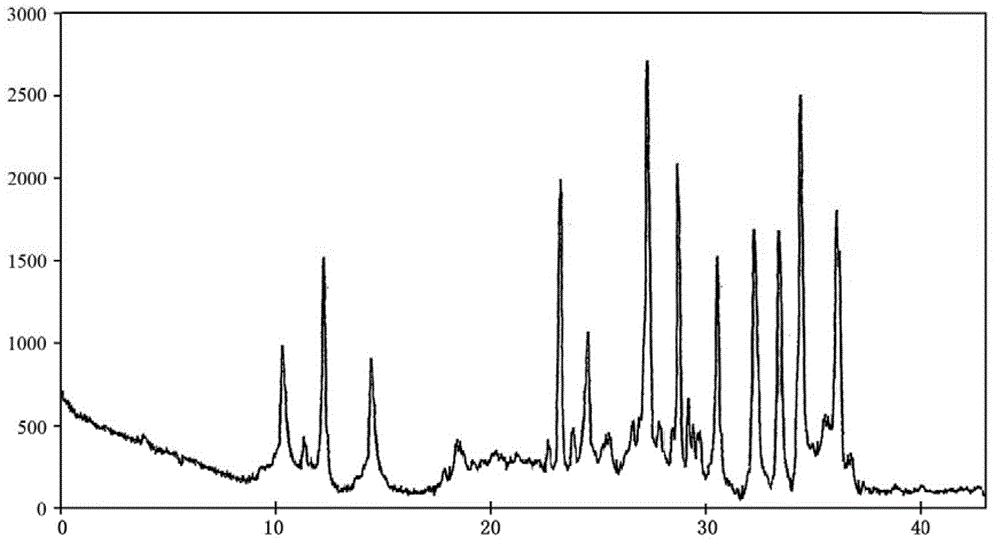
[0038] The described doxofylline crystalline compound is measured by powder X-ray diffractometry, and the X-ray powder diffraction spectrum represented by 2θ ± 0.2° diffraction angle is ...
Embodiment 2
[0039] Example 2 Preparation of doxofylline crystalline compound
[0040] (1) Take 10 g of commercially available doxofylline crude product and fully dissolve it in 55 ml of a mixed solution of water / n-butanol / acetonitrile with a volume ratio of 4:3:1.5, heat to reflux, stir and dissolve to obtain a doxofylline solution ;
[0041] (2) Add dropwise 7ml of ether / methanol solution with a volume ratio of 4:1 to the doxofylline solution under reflux and stirring, and the dripping will be completed within 8 to 10 minutes;
[0042] (3) Stop heating, stir at a rotating speed of 30rmp and cool down to 5°C, wherein the cooling rate is 5°C / 5min, let it stand for 24h to crystallize, filter, wash the filter cake with 5ml of ether, and vacuum-dry the crystalline compound of doxofylline, Yield 88.7%, HPLC 99.50%, mp178~181℃.
[0043] The described doxofylline crystalline compound is measured by powder X-ray diffractometry, and the X-ray powder diffraction spectrum represented by 2θ ± 0.2° ...
Embodiment 3
[0044] Example 3 Preparation of doxofylline crystalline compound
[0045] (1) Take 10 g of commercially available doxofylline crude product and fully dissolve it in 50 ml of a mixed solution of water / n-butanol / acetonitrile with a volume ratio of 4:3:1.5, heat to reflux, stir and dissolve to obtain a doxofylline solution ; Add 2g of activated carbon, reflux for 0.5h, filter, and collect the filtrate;
[0046](2) Add 8ml of ether / methanol solution with a volume ratio of 4:1 to the filtrate of step (1) dropwise under reflux and stirring, and it will be dripped within 8 to 10 minutes;
[0047] (3) Stop heating, stir at a rotating speed of 40rmp and cool down to 8°C, wherein the cooling rate is 6.5°C / 5min, let stand for 20h to crystallize, filter, wash the filter cake with 5ml of ether, and vacuum-dry the crystalline compound of doxofylline, Yield 88.3%, HPLC 99.79%, mp179~181℃.
[0048] The described doxofylline crystalline compound is measured by powder X-ray diffractometry, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com