Cefoxitin sodium crystal compound and cefoxitin sodium composition powder injection
A technology of cefoxitin sodium and a compound, applied in the field of medicine, can solve the problems of high price and the like, and achieve the effects of improving bioavailability, improving drug safety and good dissolution stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
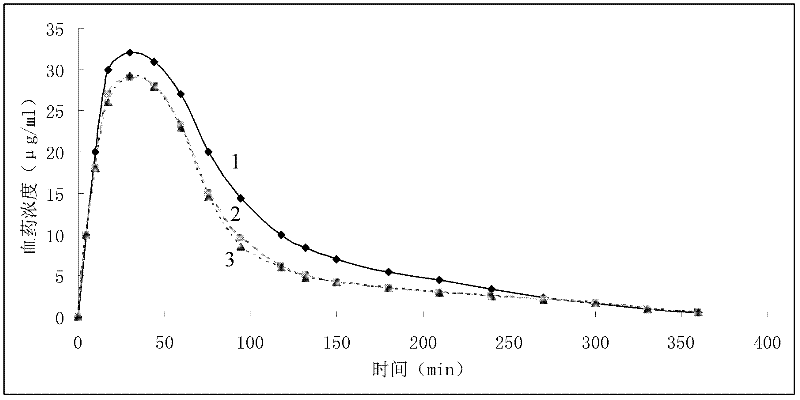
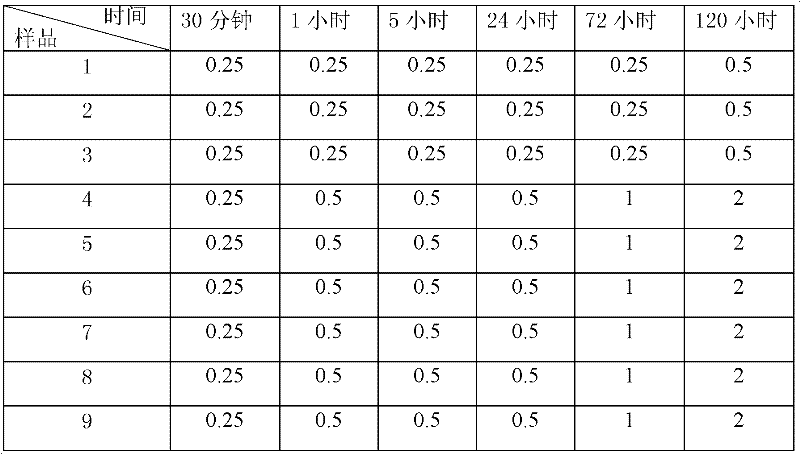
Image
Examples
Embodiment 1
[0044]Preparation of cefoxitin sodium crystalline compound: get 1 kg of cefoxitin sodium crude product, add volume ratio of 7:2.8:0.2 methanol:ethanol:water mixed solution that is 15 times of the weight of cefoxitin sodium crude product, and heat to reflux; After the crude product of cefoxitin sodium is dissolved, add activated carbon decolorizing with 0.3 times the weight of the crude product of cefoxitin sodium, and filter; Be the ethyl acetate of 0.8 times of the crude product weight of cefoxitin sodium, described stirring is 18rmp, and described dropping is to control dropping time 10 minutes and uniform speed dropwise; Cool down to 61°C for 5min, then cool down to 23°C for 10min under stirring at 10rmp, let stand for 19 hours, filter, wash twice with 6:4 methanol:ethyl acetate solution, 0.25 times each time, and dry to obtain the cephalosporin Citine sodium crystalline compound, the yield is 92.5%, and the HPLC content is 99.95%.
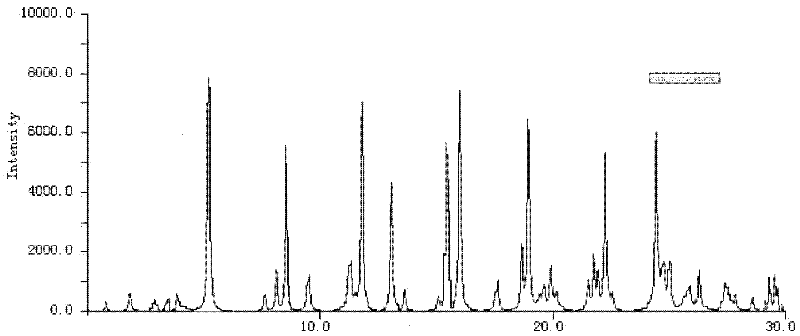
[0045] The X-ray powder diffraction pat...
Embodiment 2
[0047] Preparation of cefoxitin sodium crystalline compound: get 2 kg of cefoxitin sodium crude product, add volume ratio of 7: 2.8: 0.2 methanol: ethanol: water mixed solution that is 20 times the weight of cefoxitin sodium crude product, and heat to reflux; After the crude product of cefoxitin sodium is dissolved, add 0.3 times of the weight of the crude product of cefoxitin sodium for decolorization, filter; the filtrate is added with ethyl propionate whose volume is 1.8 times of the weight of the crude product of cefoxitin sodium, stop heating, and add dropwise the volume under stirring. Ethyl acetate that is 1.2 times the weight of the crude product of cefoxitin sodium, the stirring is 18rmp, and the dripping is to control the dripping time for 10 minutes at a constant speed; dropwise, stirring and cooling, the stirring cooling is under stirring at a rotating speed of 28rmp Cool down to 61°C for 5min, then cool down to 23°C for 10min under stirring at 10rmp, let stand for ...
Embodiment 3
[0050] Preparation of cefoxitin sodium crystalline compound: get 10 kg of cefoxitin sodium crude product, add volume ratio of 7:2.8:0.2 methanol:ethanol:water mixed solution that is 18 times of the weight of cefoxitin sodium crude product, and heat to reflux; After the cefoxitin sodium crude product is dissolved, add 0.5 times of the weight of the cefoxitin sodium crude product for decolorization, and filter; add ethyl propionate whose volume is 2.0 times the weight of the cefoxitin sodium crude product, stop heating, and add dropwise Ethyl acetate that is 1.0 times the weight of the crude product of cefoxitin sodium, the stirring is 20rmp, and the dripping is to control the dripping time for 12 minutes and drop at a constant speed; after dropping, stir and cool down, and the stirring and cooling is under stirring at a rotating speed of 29rmp. Cool down to 58°C for 5 minutes, then cool down to 25°C for 10 minutes under stirring at a rotating speed of 8rmp, let stand for 20 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com