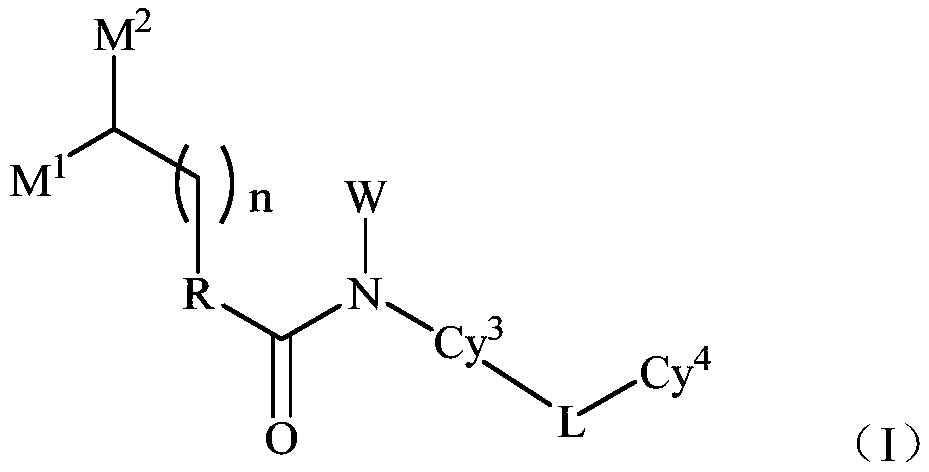
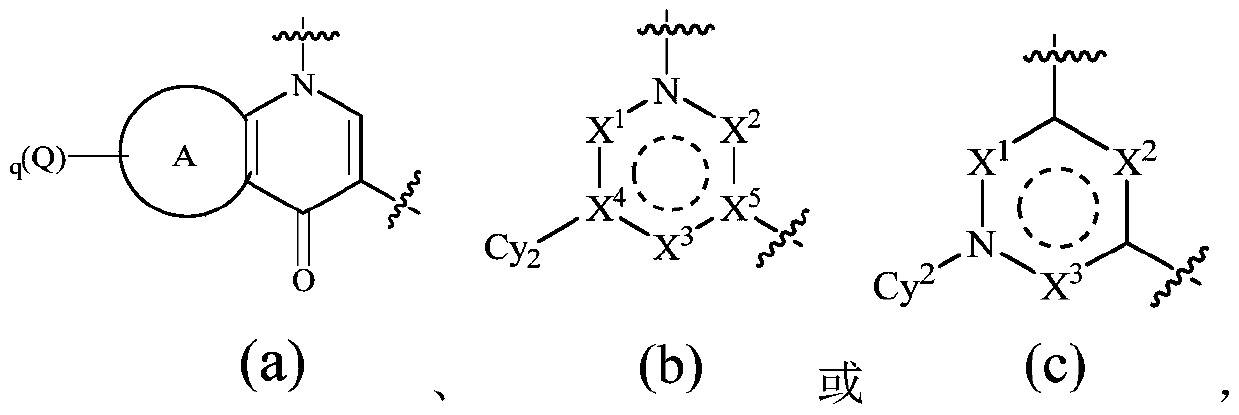
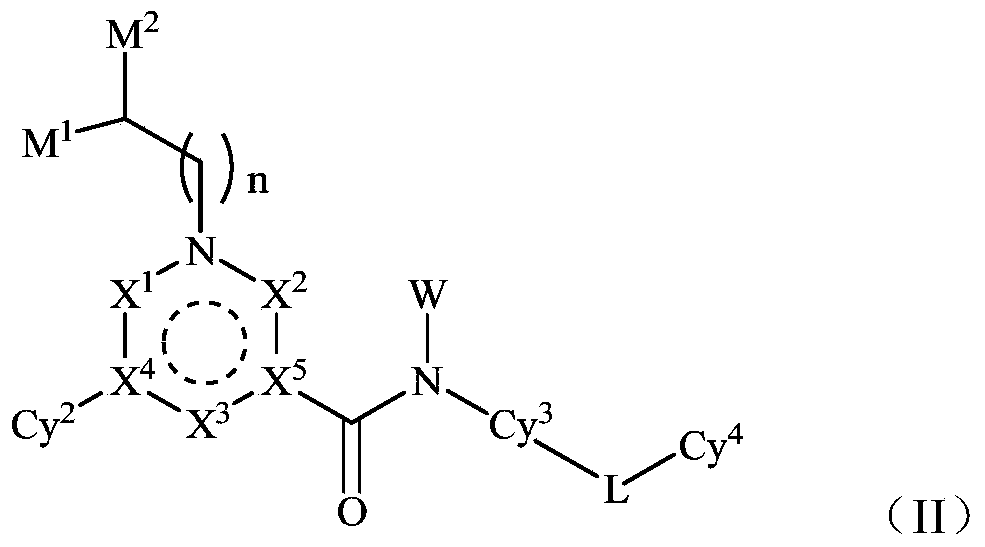
Novel quinoline derivative inhibitor
A compound and alkyl technology, applied in the field of medicine, can solve the problems of drug failure, inability to bind kinases, acquired drug resistance, etc., and achieve the effect of inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0755] Example 1: N-(5-((6,7-dimethoxyquinolin-4-yl)oxy)pyridin-2-yl)-5-(4-fluorophenyl)-1-isopropyl Synthesis of 4-oxo-1,4-dihydropyridine-3-carboxamide (compound 14)
[0756]
[0757] step:
[0758]
[0759] Step 1: Synthesis of 2-(4-fluorophenyl)acetyl chloride
[0760]
[0761] 2-(4-Fluorophenyl)acetic acid (50.0 g, 324.380 mmol, 1.0 eq) and thionyl chloride (77.18 g, 648.761 mmol, 2.0 eq) were dissolved in dichloromethane (250.0 mL), N 2 Under protection, the temperature was raised to 60°C, and refluxed for 3 hours. TLC showed that the reaction was complete. The reaction solution was concentrated under reduced pressure, and an appropriate amount of dichloromethane was added, concentrated, and repeated twice to obtain a yellow oily product.
[0762] Step 2: Synthesis of 5-(2-(4-fluorophenyl)acetyl)-2,2-dimethyl-1,3-dioxane-4,6-dione
[0763]
[0764] 2,2-Dimethyl-1,3-dioxane-4,6-dione (56.10 g, 389.3 mmol, 1.2 eq) and triethylamine (78.78 g, 7798.5 mmol, 2.4...
Embodiment 2
[0782] Example 2: N-(5-((6,7-dimethoxyquinolin-4-yl)oxy)pyridin-2-yl)-1-isopropyl-4-oxo-5-pair Synthesis of Tolyl-1,4-Dihydropyridine-3-Carboxamide (Compound 1)
[0783]
[0784] Step 1: Synthesis of ethyl 1-isopropyl-4-oxo-5-p-tolyl-1,4-dihydropyridine-3-carboxylate
[0785]
[0786] 4-oxo-5-p-tolyl-1,4-dihydropyridine-3-carboxylic acid ethyl ester (2.00g, 7.77mmol, 1.0eq), isopropyl bromide (1.15g, 9.33mmol, 1.2eq ) and K 2 CO 3 (3.22g, 23.32mmol, 3.0eq) was added into DMF (20mL), and the reaction was stirred overnight at 50°C. The completion of the reaction was monitored by TLC, filtered, the filter cake was rinsed with ethyl acetate, the filtrate was concentrated under reduced pressure, the crude product was dissolved in ethyl acetate (20 mL), washed successively with distilled water (10 mL×4) and saturated brine (10 mL), and anhydrous Dry over sodium sulfate, filter with suction, and concentrate the filtrate under reduced pressure. The crude product is purified ...
Embodiment 3
[0795] Example 3: N-(5-((6,7-dimethoxyquinolin-4-yl)oxy)pyridin-2-yl)-5-(4-fluorophenyl)-1-isopropyl Synthesis of 4-oxo-1,4-dihydropyridazine-3-carboxamide (compound 15)
[0796]
[0797] Step 1: Synthesis of 6,7-dimethoxy-4-((6-nitropyridin-3-yl)oxy)quinoline
[0798]
[0799] 6,7-dimethoxyquinolin-4-ol (15.00g, 73.10mmol, 1.0eq), 5-chloro-2-nitropyridine (11.60g, 73.10mmol, 1.0eq) and K 2 CO 3 (20.20g, 146.11mmol, 2.0eq) was added into DMF (120mL), stirred and reacted overnight at 80°C under nitrogen protection. The completion of the reaction was monitored by TLC, filtered, the filter cake was rinsed with dichloromethane, the filtrate was concentrated under reduced pressure, dissolved in dichloromethane (50mL), washed with distilled water (20×4mL) and saturated brine (20mL) successively, washed with anhydrous sulfuric acid Dry over sodium, filter with suction, and concentrate the filtrate under reduced pressure. The crude product is purified by silica gel column chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com