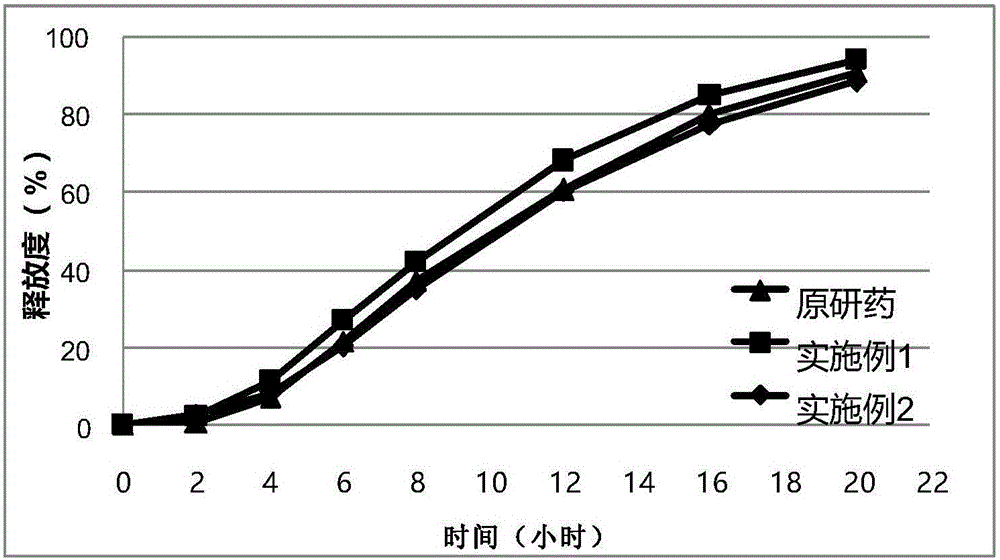
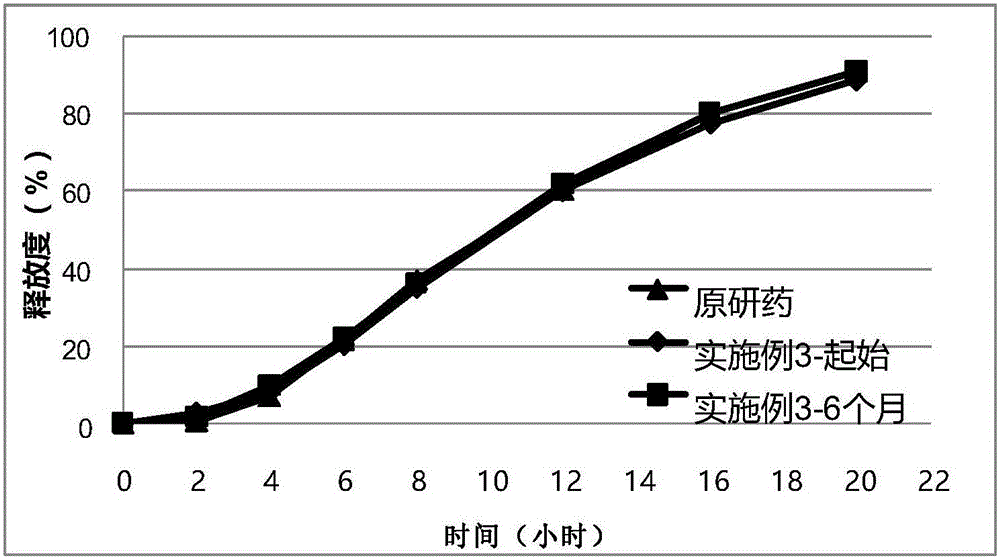
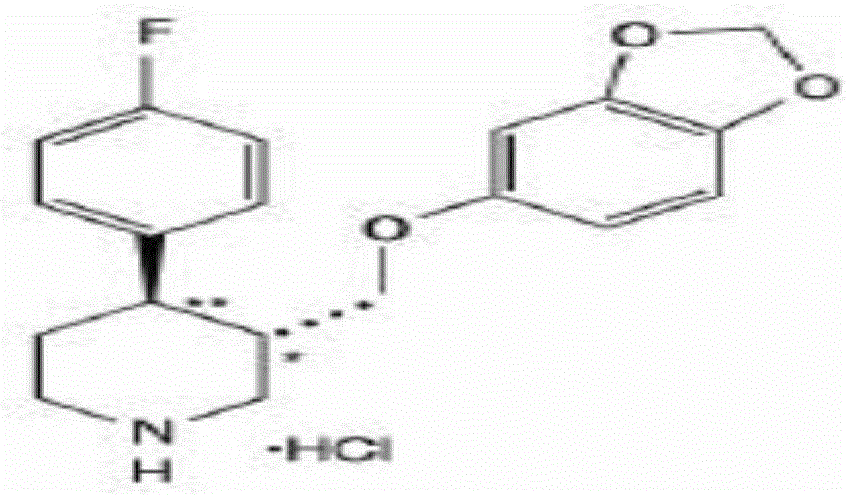
Sustained-release tablet containing dual-layer tablet core
A sustained-release tablet and double-layer tablet core technology, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, coatings, etc., can solve the problems of reducing production costs and drug prices, drug control difficulties and Risk, complex preparation process and other issues, to achieve the effect of reducing the burden of medication, complete and smooth appearance, and uniform color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The prescription is shown in Table 1:
[0065] Table 1: Prescription for 1000 tablets of paroxetine hydrochloride enteric-coated sustained-release tablets (37.5mg / tablet)
[0066]
[0067] Preparation:
[0068] 1) Preparation of the mixed powder of the drug-containing layer:
[0069] Put paroxetine hydrochloride, lactose and hydroxypropyl methylcellulose K4M in the wet mixing granulator according to the prescription amount in the above table, set the stirring speed at 150-250 rpm, and the cutting knife speed at 2000-3000 rpm After dry mixing, gradually add appropriate amount of purified water to make granules of suitable size. The prepared wet granules were moved to a fluidized bed for drying at a material temperature of 50° C., and passed through a 1.5 mm sieve to obtain granules containing paroxetine hydrochloride. The prescription amount of magnesium stearate is passed through a 20-mesh sieve and mixed evenly with the above-mentioned paroxetine hydrochloride gr...
Embodiment 2
[0077] The prescription is as shown in Table 2:
[0078] Table 2: Prescription for 1000 tablets of paroxetine hydrochloride enteric-coated sustained-release tablets (37.5mg / tablet)
[0079]
[0080]
[0081] The preparation method is the same as in Example 1.
Embodiment 3
[0083] The prescription is shown in Table 3:
[0084] Table 3: Prescription for 1000 tablets of paroxetine hydrochloride enteric-coated sustained-release tablets (12.5mg / tablet)
[0085]
[0086] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com