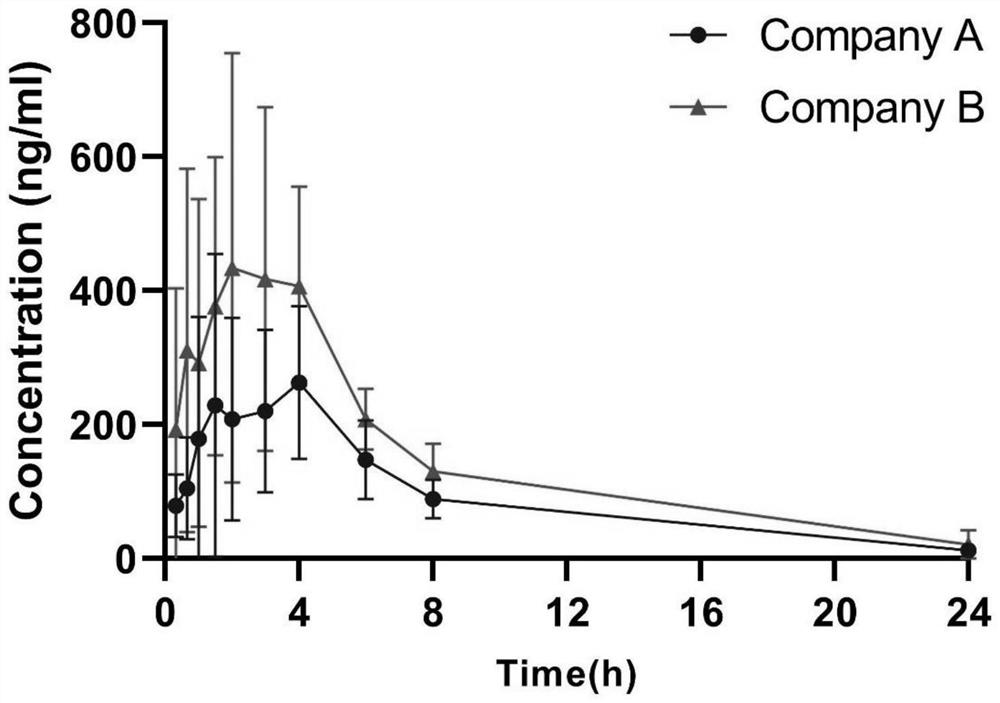
Chlortetracycline hydrochloride dry suspension as well as preparation method and application thereof
A technology of chlortetracycline hydrochloride and dry suspension is applied in the field of chlortetracycline hydrochloride dry suspension and its preparation, and achieves the effects of good clinical effect, good palatability and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Suspension formulation screening:
[0040] 1. Orthogonal experimental design
[0041] 1.1 Instruments: electronic balance (sensitivity 0.001g), water bath, digital high-speed dispersion homogenizer, beaker, 100ml measuring cylinder.
[0042] 1.2 Raw materials: aureomycin hydrochloride, xanthan gum, crospovidone, hydroxypropyl methylcellulose, ethyl vanillin, citric acid.
[0043] 1.3 Method: Accurately weigh 22g of aureomycin hydrochloride crushed through a 200-mesh sieve, put it in a 1000ml beaker, then add suspending agent, 5g of citric acid, 0.5g of ethyl vanillin, and purified water 500ml. Put the beaker into a water bath, and process it with a digital display high-speed dispersing homogenizer for 30 minutes, maintaining the temperature of the suspension in the beaker at 40°C.
[0044] 1.4 Orthogonal test design: On the basis of searching literature and previous preliminary tests, an orthogonal test was carried out with xanthan gum, crospovidone, hydroxypropyl me...
Embodiment 2
[0054]
[0055] The preparation method is as follows:
[0056] Aureomycin hydrochloride is pulverized with a pin-disc pulverizer, and passed through a 200 mesh sieve; the remaining auxiliary materials are respectively passed through a 120 mesh sieve;
[0057] Weigh aureomycin hydrochloride, xanthan gum, polyvinylpyrrolidone K30, hydroxypropyl methylcellulose, and citric acid according to the proportion and put them into the stirring tank with vacuum, add ethyl vanillin directly at the gate, and start the stirring tank Stir the motor, add 500L of purified water, and then start the upper and lower suction high-shear emulsifiers, throttling upper suction emulsifiers, keep the tank temperature at 35-40°C, high-speed shear, and disperse for 30 minutes to form a suspension;
[0058] Spray-dry the suspension to make fine particles and pass through an 80-mesh sieve. The spray drying air inlet temperature is controlled at 120°C, and the outlet air temperature is controlled at 80°C;...
Embodiment 3
[0061]
[0062] The preparation method is as follows:
[0063] Aureomycin hydrochloride is pulverized with a pin-disc pulverizer, and passed through a 200 mesh sieve; the remaining auxiliary materials are respectively passed through a 120 mesh sieve;
[0064] Weigh aureomycin hydrochloride, xanthan gum, polyvinylpyrrolidone K30, hydroxypropyl methylcellulose, and citric acid according to the proportion and put them into the stirring tank with vacuum, add ethyl vanillin directly at the gate, and start the stirring tank Stir the motor, add 500L of purified water, and then start the upper and lower suction high-shear emulsifiers, throttling upper suction emulsifiers, keep the tank temperature at 35-40°C, high-speed shear, and disperse for 30 minutes to form a suspension;
[0065] Spray-dry the suspension to make fine particles and pass through an 80-mesh sieve. The spray drying air inlet temperature is controlled at 120°C, and the outlet air temperature is controlled at 80°C;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com