Uses of valnemulin hydrogen tartrate in veterinary drugs
A technology of warnimulin and tartaric acid, which is applied in the direction of antibacterial drugs and active ingredients of esters, can solve the problems of increased cost and no warnimulin tartrate, etc., and achieves easy storage, small residual amount and good therapeutic effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
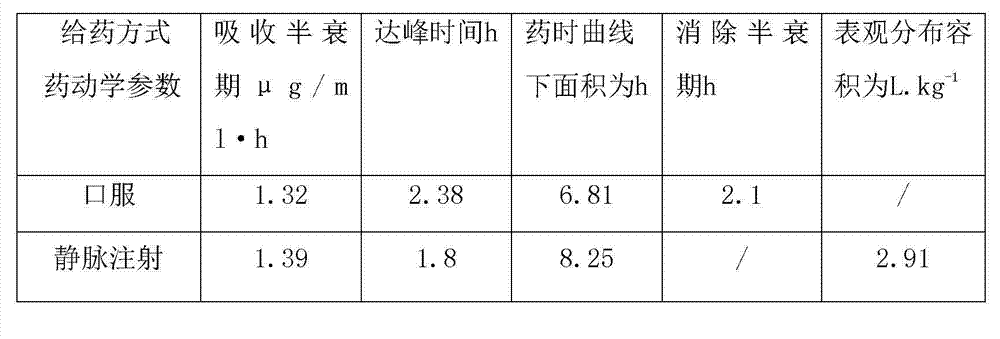
[0031] Embodiment 5 is the pharmacokinetic test of warnimulin tartrate.
[0032] In this study, high performance liquid chromatography was used to study the pharmacokinetic characteristics of a single dose of warnemulin tartrate (12mg / kg body weight) in pigs through oral and intravenous injection.
[0033] The plasma drug extraction method uses acetonitrile as the plasma protein precipitant and extraction agent, then adds anhydrous sodium sulfate to mix and centrifuge, then takes the supernatant and blows it dry, and the residue is ultrasonically dissolved in the mobile phase for HPLC detection. With tiamulin fumarate as the internal standard, the concentration range of 0.05μg / ml to 5μg / ml of warnimulin tartrate, the chromatographic peak area and concentration showed a good linear relationship, and the correlation coefficient reached more than 0.996. The limit of quantification of Lin in plasma is 0.05μg / ml.
[0034] Compartment model analysis showed that the data in the oral...
Embodiment 6
[0038] Embodiment 6 is the residual elimination test of warnemulin tartrate premix.
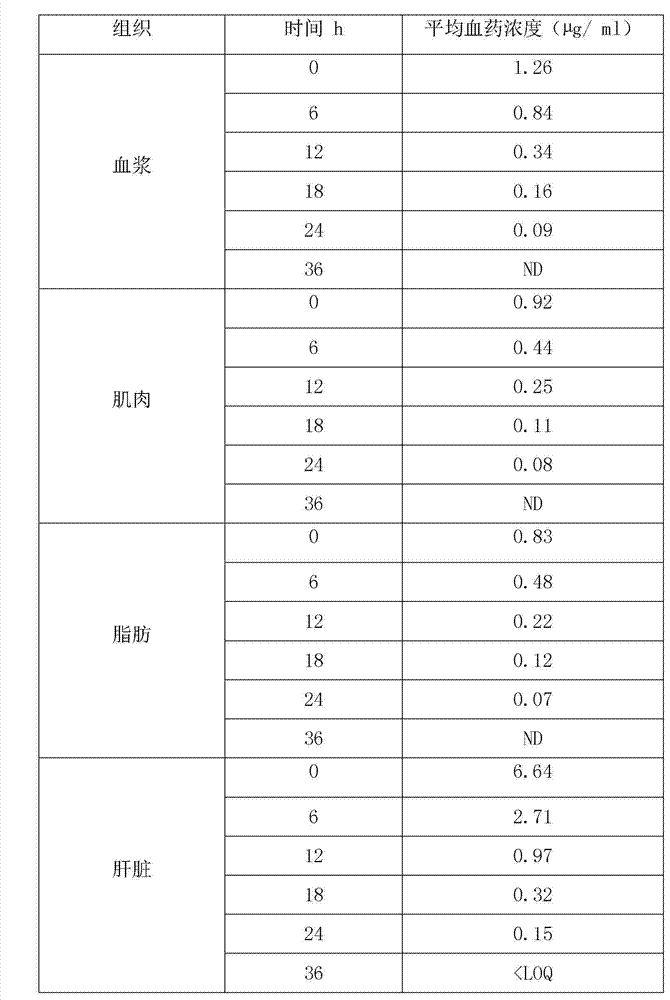
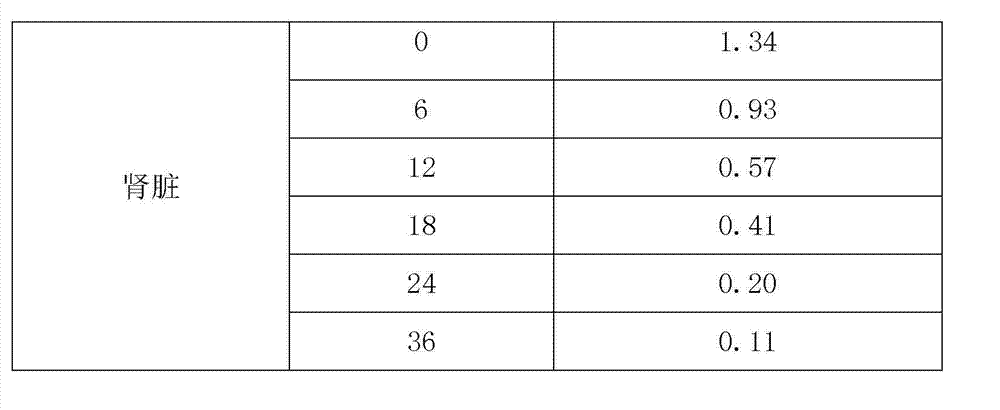
[0039] In this experiment, the elimination rule of warnemulin tartrate residues in pigs was studied. The samples were quantified by internal standard method, and pre-treated by liquid-liquid extraction, nitrogen drying, concentration and other steps. Liquid chromatography (RP-HPLC) determination. In the study of drug residues, pigs were continuously fed with a dose of 240 g of warnemulin tartrate per ton of feed mixed with warnemulin tartrate premix for 21 days. , 18, 24, 36h collected plasma, muscle, fat, liver and kidney samples for analysis and determination.
[0040] Give pigs 240g of warnemulin tartrate per ton of feed, and continuously feed mixed warnemulin tartrate premix for 21 days. The coefficient and elimination half-life are shown in Table-3. The results show that the drug concentration and elimination rate are different in different tissues. The concentration in the liver is the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com