Toltrazuril potassium salt soluble powder as well as preparation method and usage thereof
A toltrazuril and soluble technology, which is applied in powder delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the unfavorable production, transportation and use of toltrazuril raw material drugs, and different mixing and administration methods. Convenience, inaccurate dosage and other problems, to achieve the effect of good solubility, small adverse reactions and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
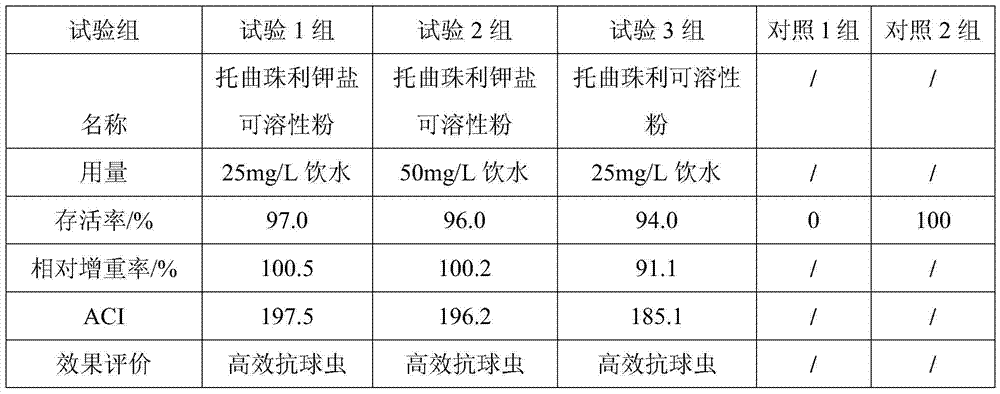
Examples
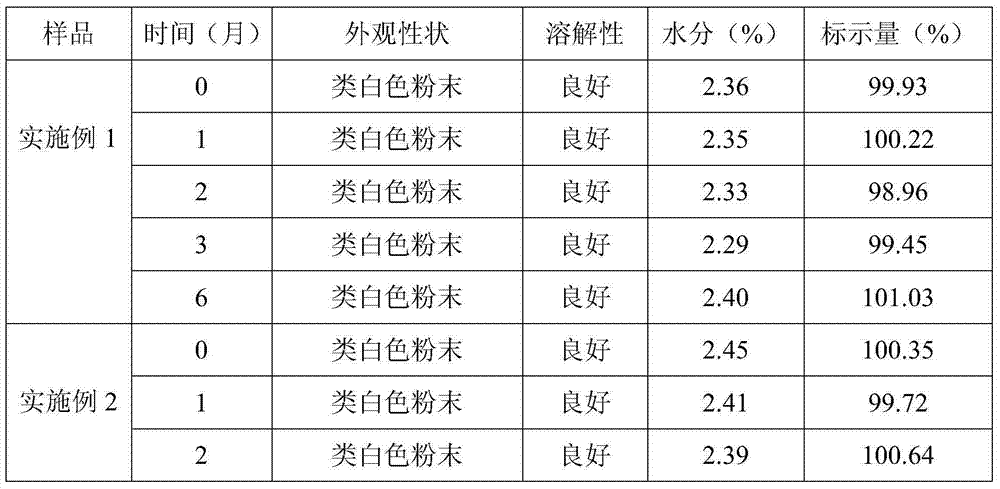
Embodiment 1
[0023] 1) Weigh 5.0kg of toltrazuril potassium salt, 0.5kg of anhydrous sodium bicarbonate, 1.0kg of anhydrous sodium carbonate, 0.2kg of anhydrous calcium chloride, 37.4kg of lactose and 55.9kg of mannitol. Sodium hydrogen and anhydrous sodium carbonate are uniformly mixed as a pH regulator, lactose and mannitol are uniformly mixed as a water-soluble diluent, lactose can also be replaced by sucrose and glucose, and mannitol can also be replaced by sorbitol. ;
[0024] 2) Weigh 5.0 kg of water-soluble diluent and tortrazuril potassium salt and mix well to obtain a mixture A of water-soluble diluent and toltrazuril potassium salt;
[0025] 3) Dilute the remaining water-soluble diluent with the doubling dilution method to dilute the anhydrous calcium chloride and the pH regulator once respectively to obtain the mixture B of the water-soluble diluent and anhydrous calcium chloride, the water-soluble diluent and the pH regulator mixture C;
[0026] 4) Add mixture A, mixture B, m...
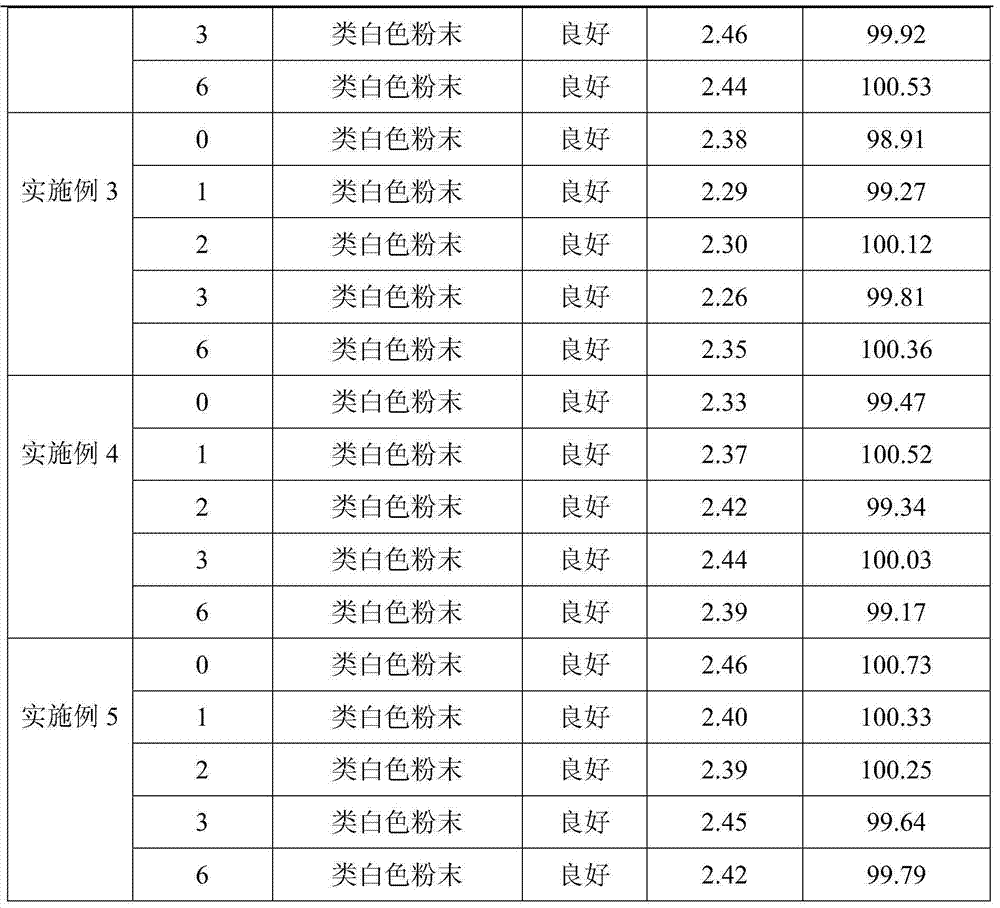
Embodiment 2
[0028] 1) Weigh 10.0kg of toltrazuril potassium salt, 1.0kg of anhydrous sodium bicarbonate, 2.5kg of anhydrous sodium carbonate, 0.5kg of hydrogenated tallow primary amine, 21.6kg of lactose and 64.4kg of mannitol. Sodium hydrogen and anhydrous sodium carbonate are uniformly mixed as a pH regulator, and lactose and mannitol are uniformly mixed as a water-soluble diluent;
[0029] 2) Weigh 10.0 kg of water-soluble diluent and tortrazuril potassium salt and mix well to obtain a mixture A of water-soluble diluent and toltrazuril potassium salt;
[0030] 3) Dilute the remaining water-soluble diluent with the double dilution method to dilute the hydrogenated tallow primary amine and the pH regulator once, respectively to obtain the mixture B of the water-soluble diluent and the hydrogenated tallow primary amine, the water-soluble diluent and the pH regulator mixture C;
[0031] 4) Add mixture A, mixture B, mixture C and the remaining water-soluble diluent after dilution in step 3...
Embodiment 3
[0033] 1) Weigh 20.0kg of toltrazuril potassium salt, 1.5kg of anhydrous sodium bisulfite, 4.0kg of anhydrous sodium sulfite, 0.8kg of ammonium chloride and 73.7kg of glucose, first mix anhydrous sodium bisulfite and anhydrous sodium sulfite Mix well as a pH regulator, glucose can also be replaced by lactose;
[0034] 2) Weigh 20.0 kg of anhydrous glucose and toltrazuril potassium salt and mix well to obtain a mixture A of anhydrous glucose and toltrazuril potassium salt;
[0035] 3) Dilute the remaining glucose with ammonium chloride and pH regulator once each by the doubling dilution method to obtain mixture B of glucose and ammonium chloride and mixture C of glucose and pH regulator;
[0036] 4) Add mixture A, mixture B, mixture C and the remaining anhydrous glucose diluted in step 3) into the three-dimensional mixer, start the mixer, and mix thoroughly for 45 minutes to obtain toltrazuril potassium salt soluble powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com