Synthesis method for valnemulin hydrochloride
A technology of vonimulin hydrochloride and a synthesis method, which is applied in the preparation of thioethers, organic chemistry and other directions to achieve the effects of strong antibacterial activity, clear antibacterial mechanism and low residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Dissolve 75.7g (0.2mol) of pleuromutilin and 42g (0.22mol) of p-toluenesulfonyl chloride in a mixed solution of 200ml of methyl tert-butyl ether and 40mL of distilled water. mol / ml NaOH solution 50ml. Then it was heated to reflux while vigorously stirring. After 20 minutes, a large amount of white matter was formed, and it was stirred for another 20 minutes. The resulting white solid was filtered with a Buchner funnel, washed with methyl tert-butyl ether and distilled water, and dried naturally to obtain a white powder product with a yield of 97.8%.
Embodiment 2
[0045] Dissolve 37.9g (0.1mol) of pleuromutilin and 22.9g (0.12mol) of p-toluenesulfonyl chloride in a mixed solution of 80ml of methyl tert-butyl ether and 20mL of distilled water, and slowly stir the mixture and drop it into 30ml of 0.01mol / ml NaOH solution. Then it was heated to reflux while vigorously stirring. After 30 minutes, a large amount of white matter was formed, and it was stirred for another 20 minutes. The resulting white solid was filtered with a Buchner funnel, rinsed with methyl tert-butyl ether and distilled water, and dried naturally to obtain a white powder product with a yield of 97.1%.
Embodiment 3
[0047] Dissolve 37.9g (0.1mol) of pleuromutilin and 20.9g (0.11mol) of p-toluenesulfonyl chloride in a mixed solution of 100ml of methyl tert-butyl ether and 25mL of distilled water, and slowly stir the mixture and drop it into 30ml of 0.01mol / ml NaOH solution. Then it was heated to reflux while vigorously stirring. After 30 minutes, a large amount of white matter was formed, and it was stirred for another 20 minutes. The resulting white solid was filtered with a Buchner funnel, washed with methyl tert-butyl ether and distilled water, and dried naturally to obtain a white powder product with a yield of 96.8%.
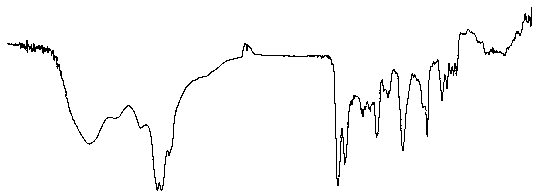
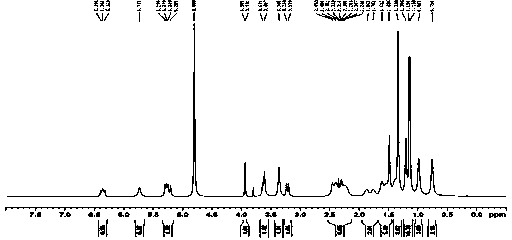
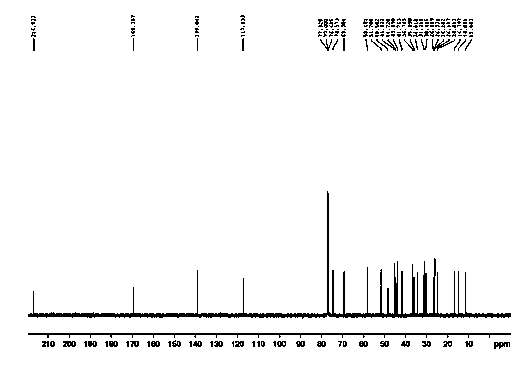
[0048] mp 147~148 oC; IR (KBr): 3446, 2924, 2863, 1732, 1633, 1597, 1456, 1371, 1297, 1233, 1117, 1035, 832, 664, 560 cm-1; 1H NMR (400 MHz, CDCl3) δ 0.63 (d, 3H, J = 6.8 Hz), 0.87 (d, 3H, J = 6.8Hz), 1.11–1.15 (m, 1H), 1.22-1.26 (s, 5H), 1.33–1.36 (m , 1H), 1.41–1.44 (m, 1H), 1.46-1.50 (m, 5H), 1.63-1.65 (dd, 2H,J1=10Hz,J2=7.2 Hz), 2.01–2.08(m, 3H), 2.21 –2.29 (m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com