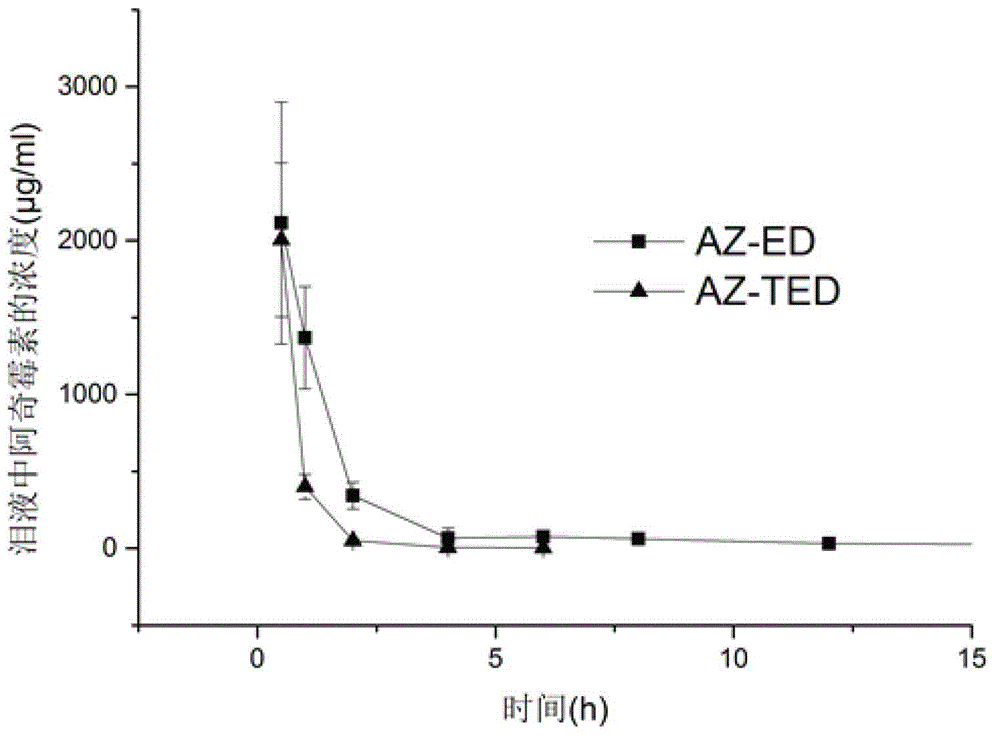
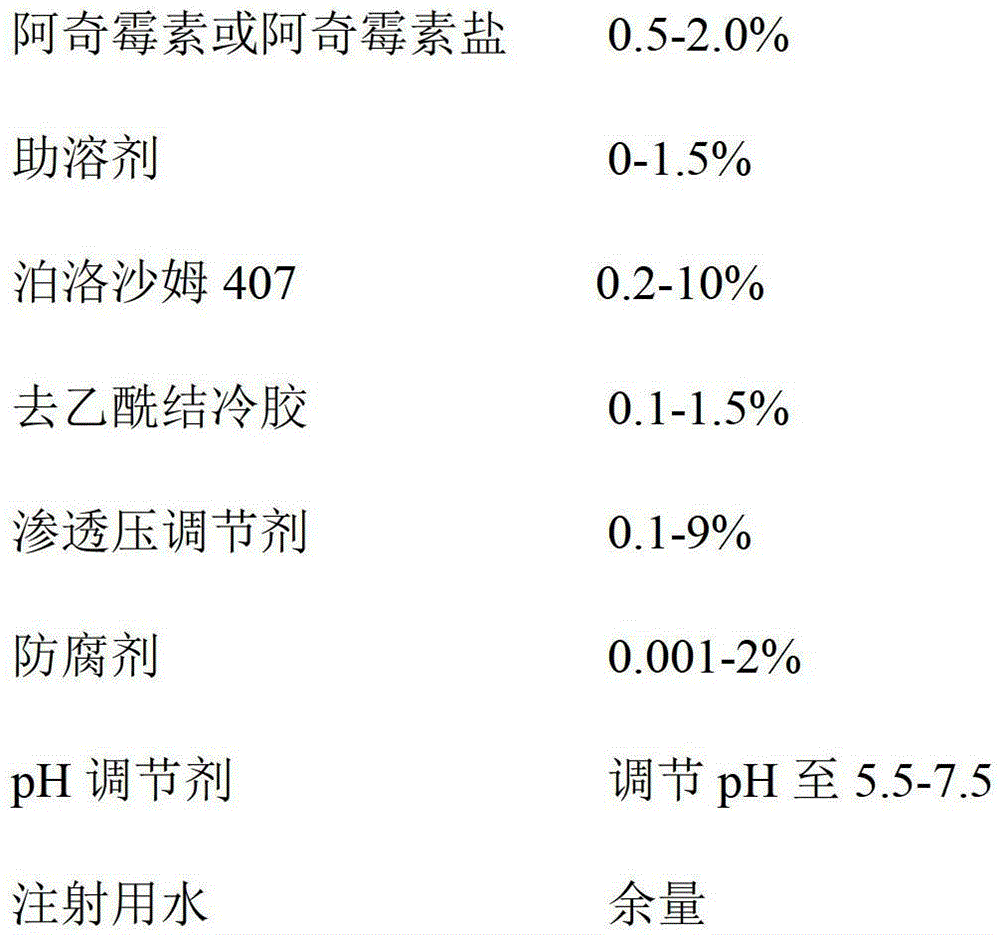
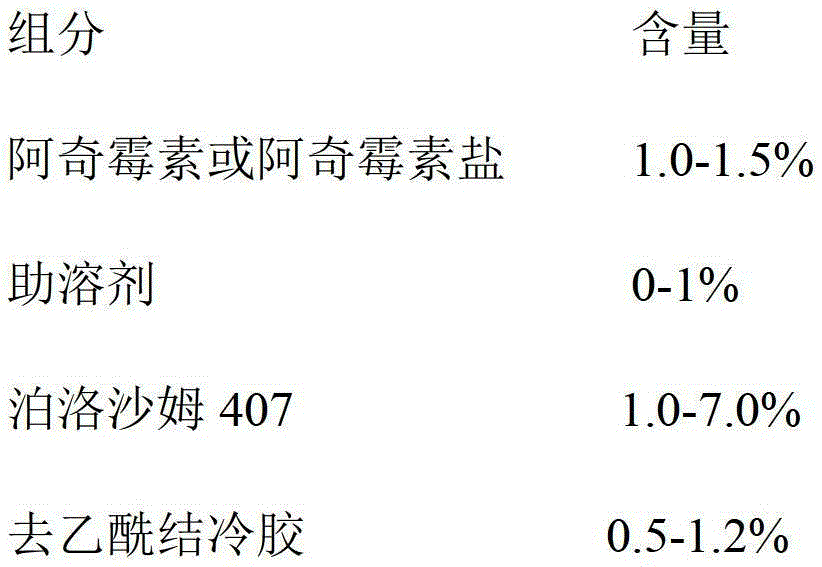
Azithromycin eye drops and preparation method thereof
A technology of azithromycin and eye drops, applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as aging, and achieve the effects of low toxicity, long treatment time, and few systemic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] formula:
[0042]
[0043] Preparation Process:
[0044] Weigh citric acid according to the prescription amount, add water for injection to dissolve, the weight dosage of water for injection is 20% of the total weight of water for injection, then add the prescription amount of azithromycin to make it completely dissolved, then add poloxamer 407, at 4 ℃ Let it swell completely and set aside;
[0045] Take the deacetylated gellan gum, mannitol and benzalkonium chloride of the prescribed amount, add water for injection to dissolve, the weight dosage of water for injection is 70% of the total weight of water for injection, keep warm at 40°C, and set aside;
[0046] Combine them, adjust the pH to 6 with 1 mol / L trishydroxymethylaminomethane, supplement the remaining amount of water for injection, stir evenly, filter through a 0.22 μm microporous membrane, and pack in separate packages to obtain the product.
Embodiment 2
[0048]
[0049] Preparation Process:
[0050] Weigh citric acid according to the prescription amount, add water for injection to dissolve, the weight dosage of water for injection is 30% of the total weight of water for injection, then add the prescription amount of azithromycin to make it completely dissolved, then add poloxamer 407, at 4 ℃ Let it swell completely and set aside;
[0051] Take the deacetylated gellan gum, mannitol and benzalkonium bromide of the prescription, add water for injection to dissolve, the weight dosage of water for injection is 60% of the total weight of water for injection, keep warm at 40°C, and set aside;
[0052] Combine them, adjust the pH to 6.5 with 1 mol / L trishydroxymethylaminomethane, add the remaining amount of water for injection, stir evenly, filter through a 0.22 μm microporous membrane, and pack in separate packages to obtain the product.
Embodiment 3
[0054]
[0055]
[0056] Preparation Process:
[0057] Weigh citric acid according to the prescription amount, add water for injection to dissolve, the weight dosage of water for injection is 20% of the total weight of water for injection, then add the prescription amount of azithromycin to make it completely dissolved, then add poloxamer 407, at 4 ℃ Let it swell completely and set aside;
[0058] Take the deacetylated gellan gum, mannitol and benzalkonium chloride of the prescribed amount, add water for injection to dissolve, the weight dosage of water for injection is 70% of the total weight of water for injection, keep warm at 40°C, and set aside;
[0059] Combine them, adjust the pH to 5.5 with 1 mol / L trishydroxymethylaminomethane, add the remaining amount of water for injection, stir evenly, filter through a 0.22 μm microporous membrane, and pack in separate packages to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com