Patents
Literature
41results about How to "Small increment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
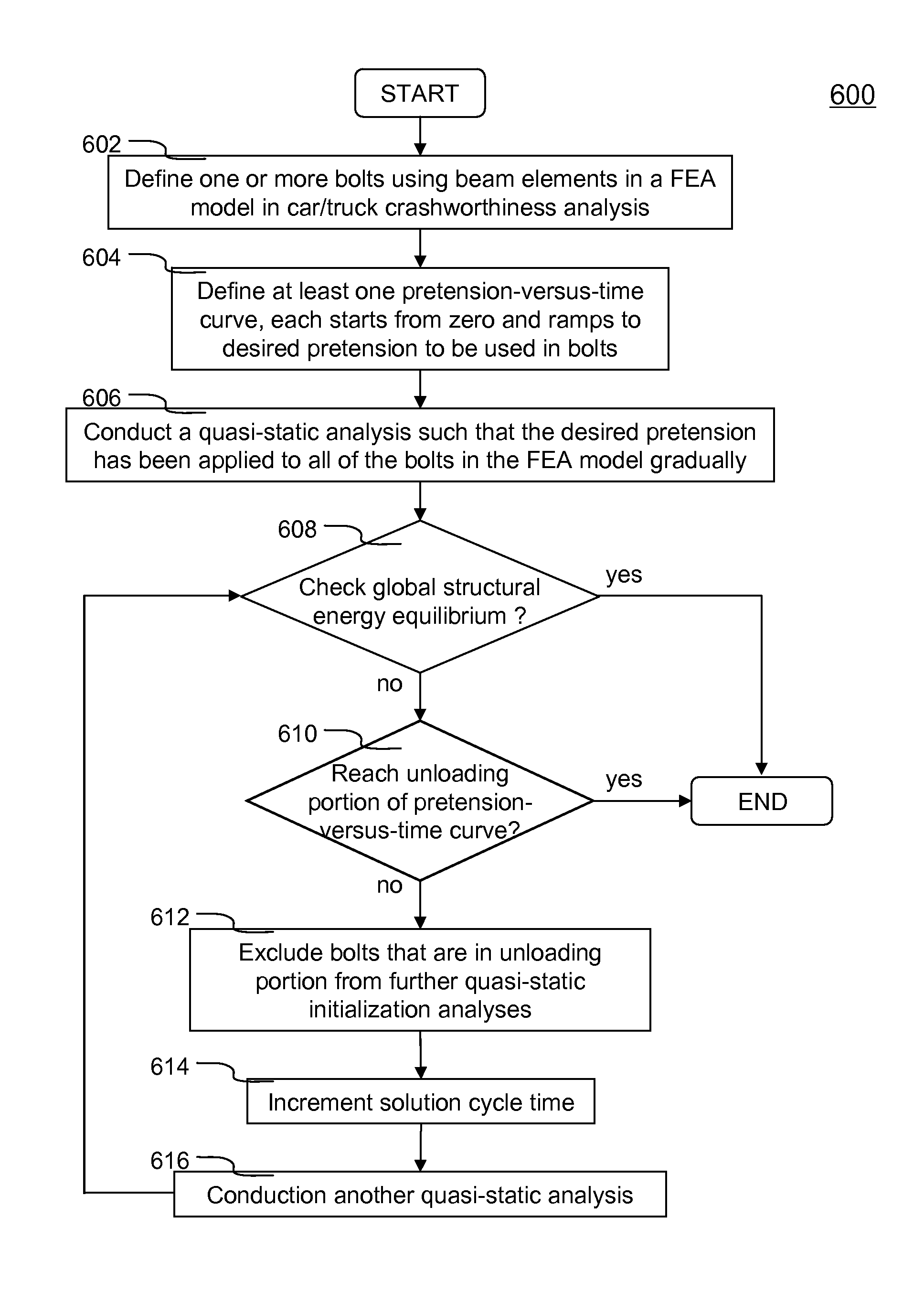
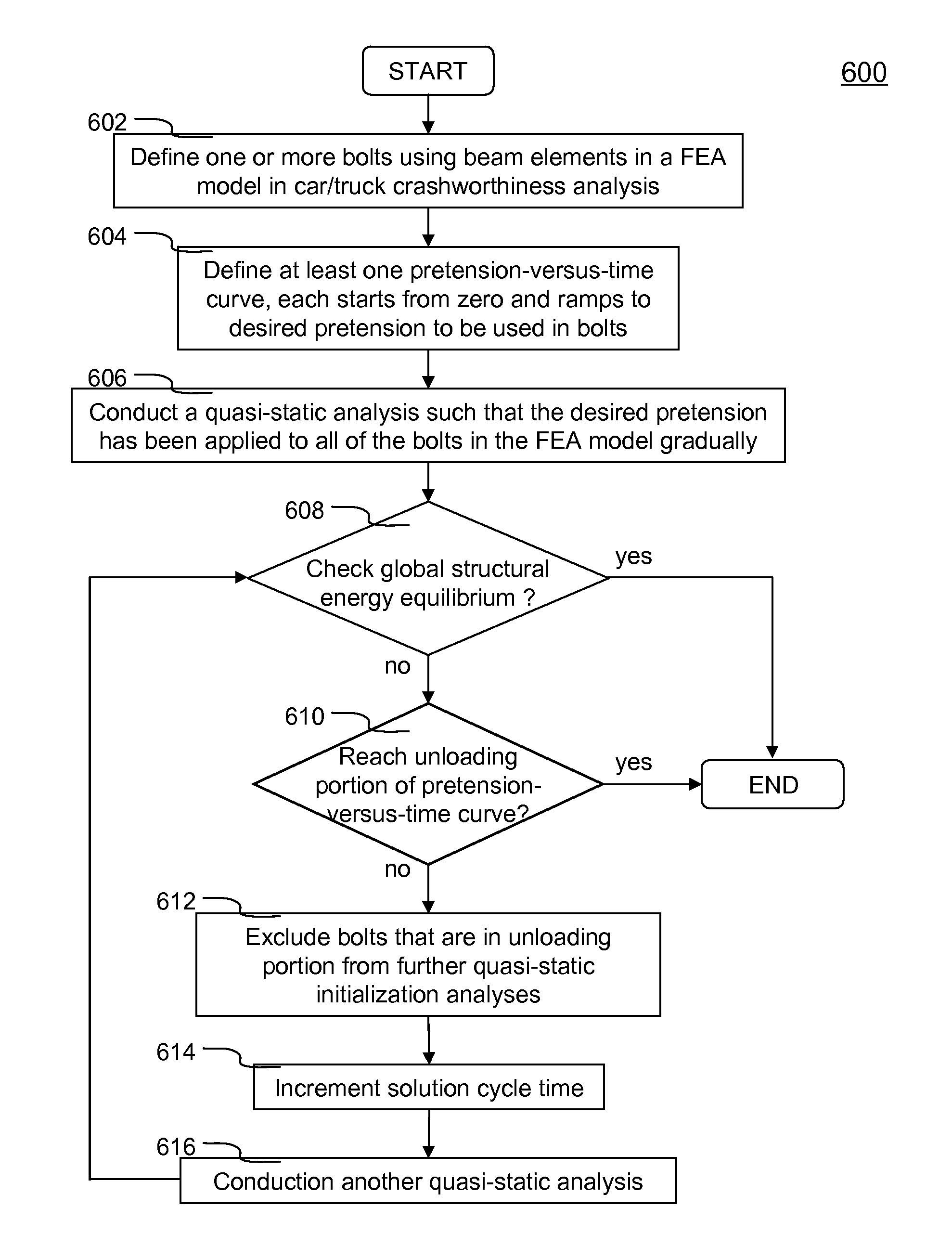
Method of initializing bolt pretension in a finite element analysis
ActiveUS8069017B2Small incrementAvoid the needDesign optimisation/simulationSpecial data processing applicationsElement analysisEngineering
In one aspect of the invention, each bolt is modeled using a beam element in a FEA model. To apply desired pretension to one or more bolts, at least one pretension-versus-time curve is specified. Each pretension-versus-time curve includes ramp portion, desired pretension portion and optional unloading portion. Duration of the pretension-versus-time curve generally covers first 0.5-1% of total simulation time of a car crashworthiness analysis. Ramp portion starts from zero to desired pretension in a substantially linear manner, and hence being configured for applying desired pretension to a bolt gradually with smaller increments. Desired pretension portion is configured for ensuring the desired pretension can actually be applied to the beam element during an initialization process—a series of quasi-static analyses. Since the method is independent of the deformation of the beam, the method completely avoids the need to iteratively determine an axial strain or displacement that gives the desired pretension.
Owner:ANSYS
Apparatus and method of converting image signal for four-color display device, and display device including the same
InactiveUS20050219274A1Increase valueSmall incrementTelevision system detailsColor signal processing circuitsSignal classificationDisplay device
A method of converting image signals for a display device including six-color subpixels is provided, which includes: classifying three-color input image signals into maximum, middle, and minimum; decomposing the classified signals into six-color components; determining a maximum among the six-color components; calculating a scaling factor; and extracting six-color output signals.
Owner:SAMSUNG DISPLAY CO LTD
Lamination stack with center interlock
InactiveUS6984913B2Quick laminationFirmly connectedManufacturing stator/rotor bodiesMagnetic circuit shape/form/constructionEngineering
Owner:L H CARBIDE
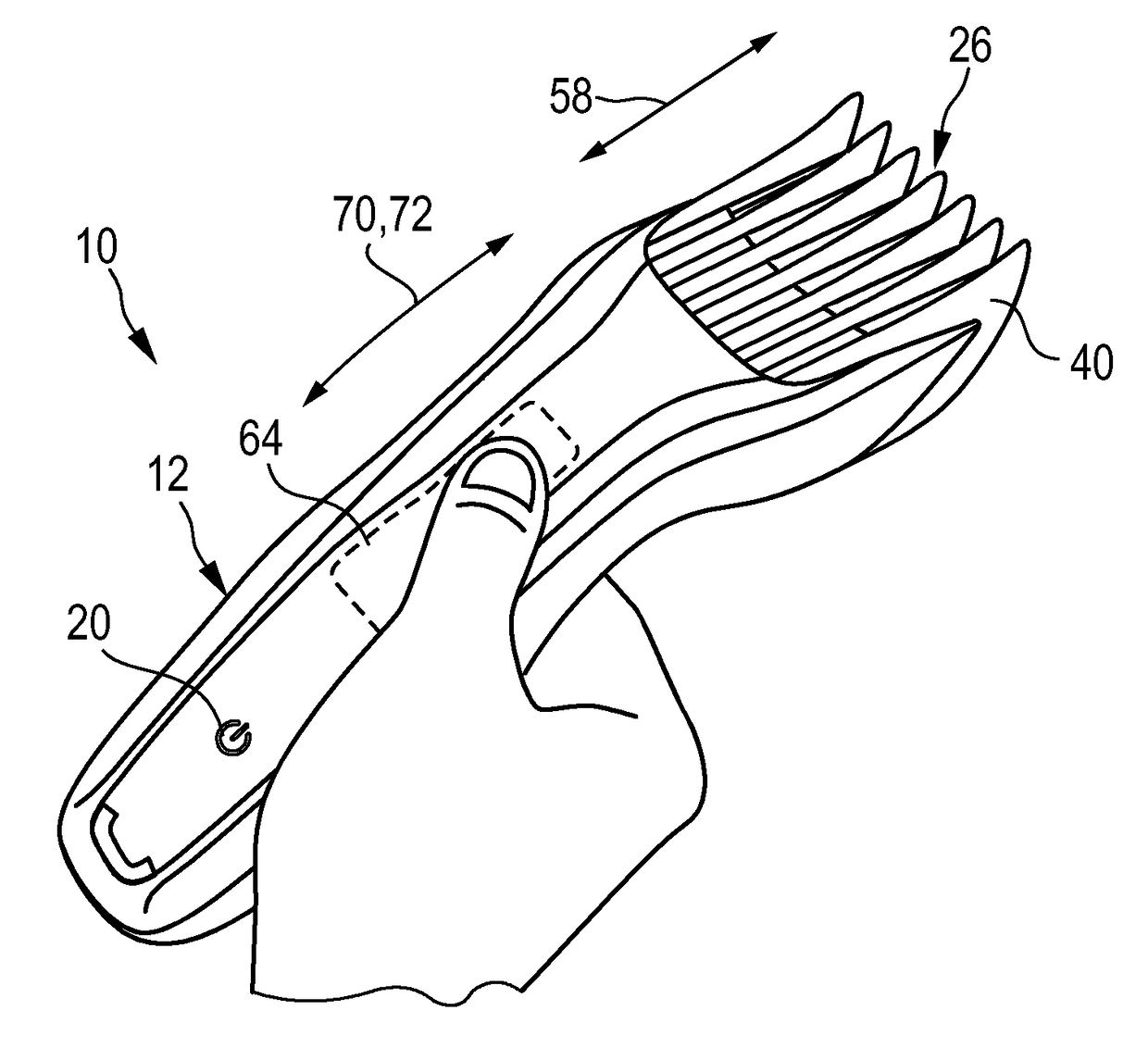
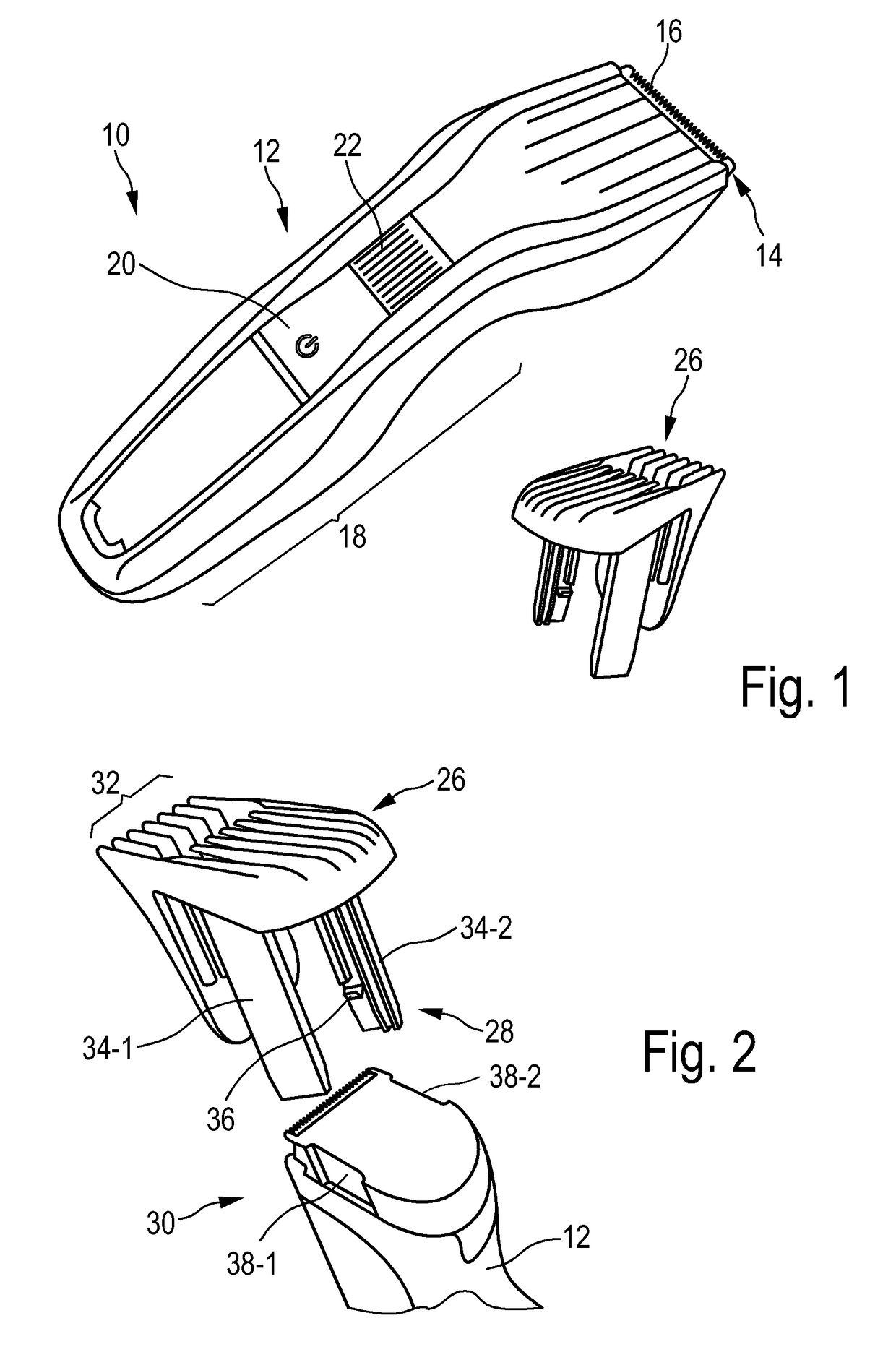
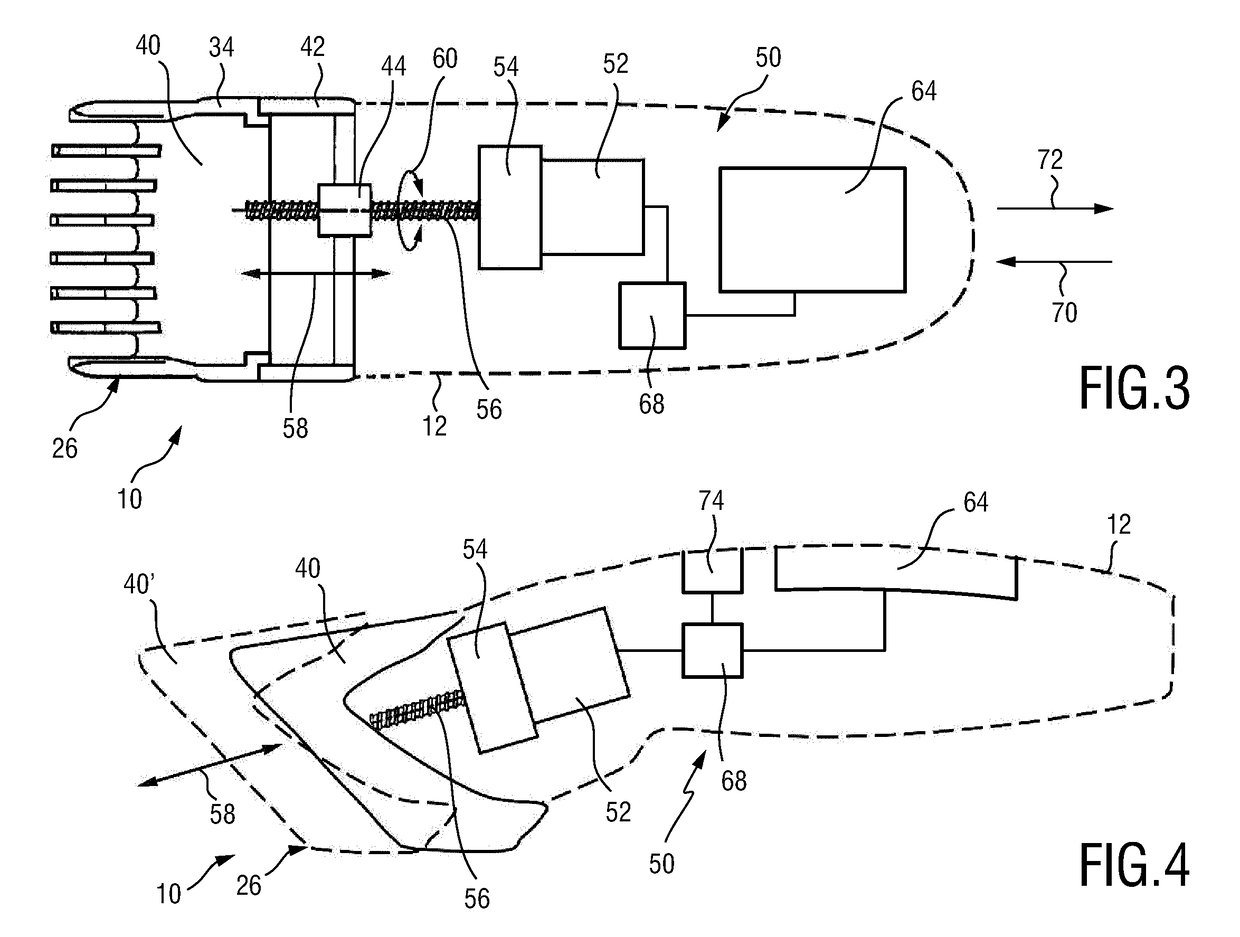
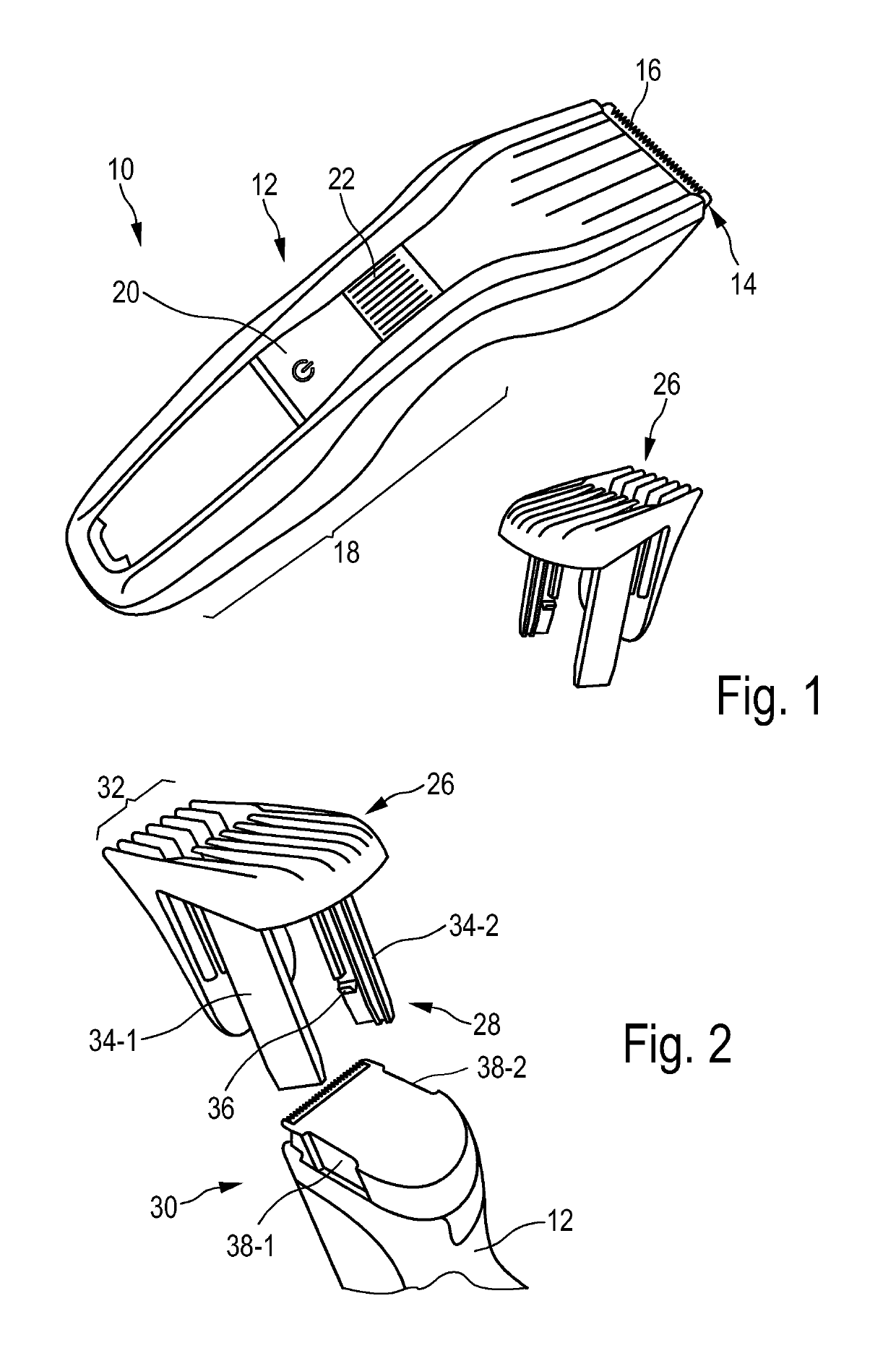
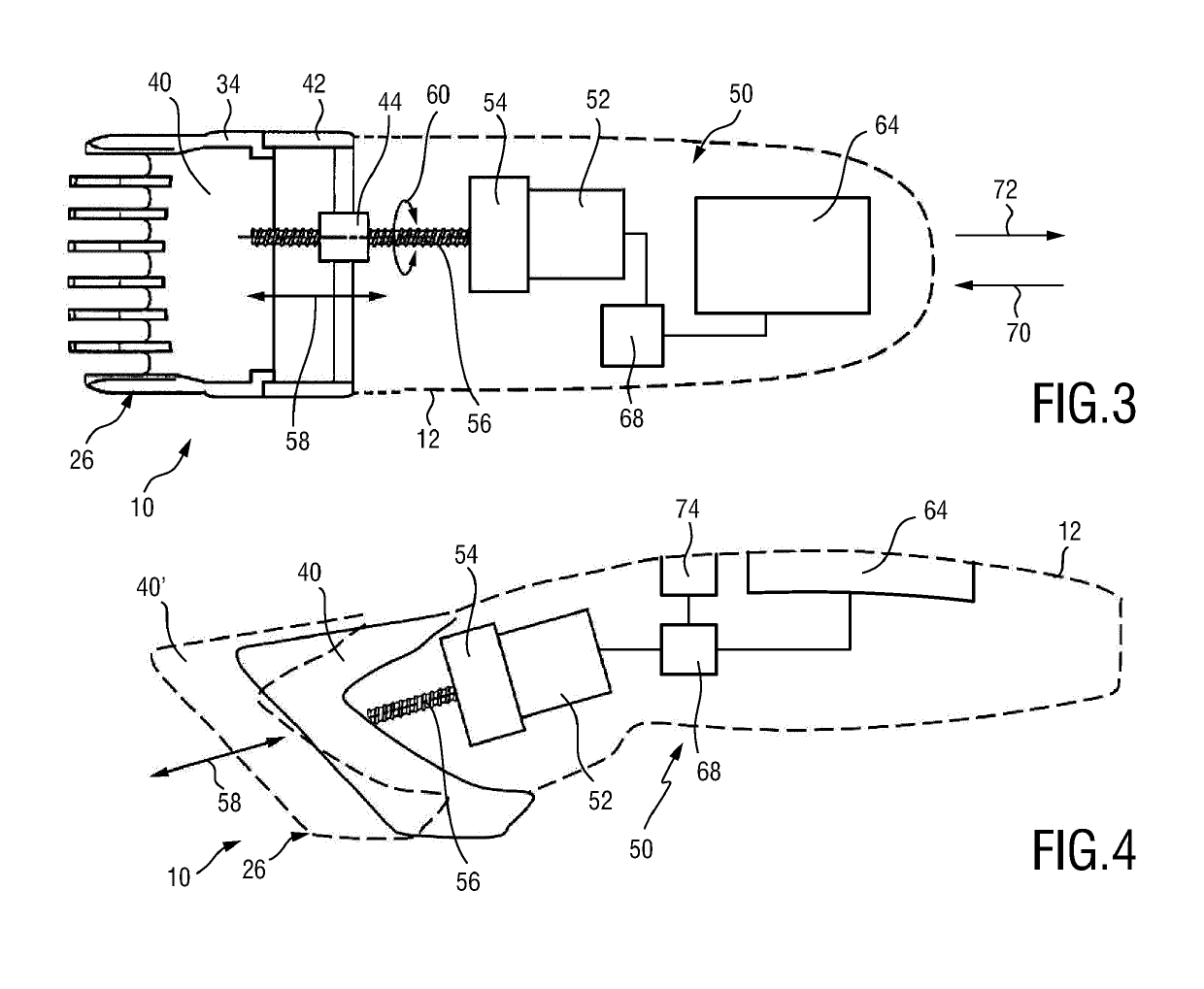
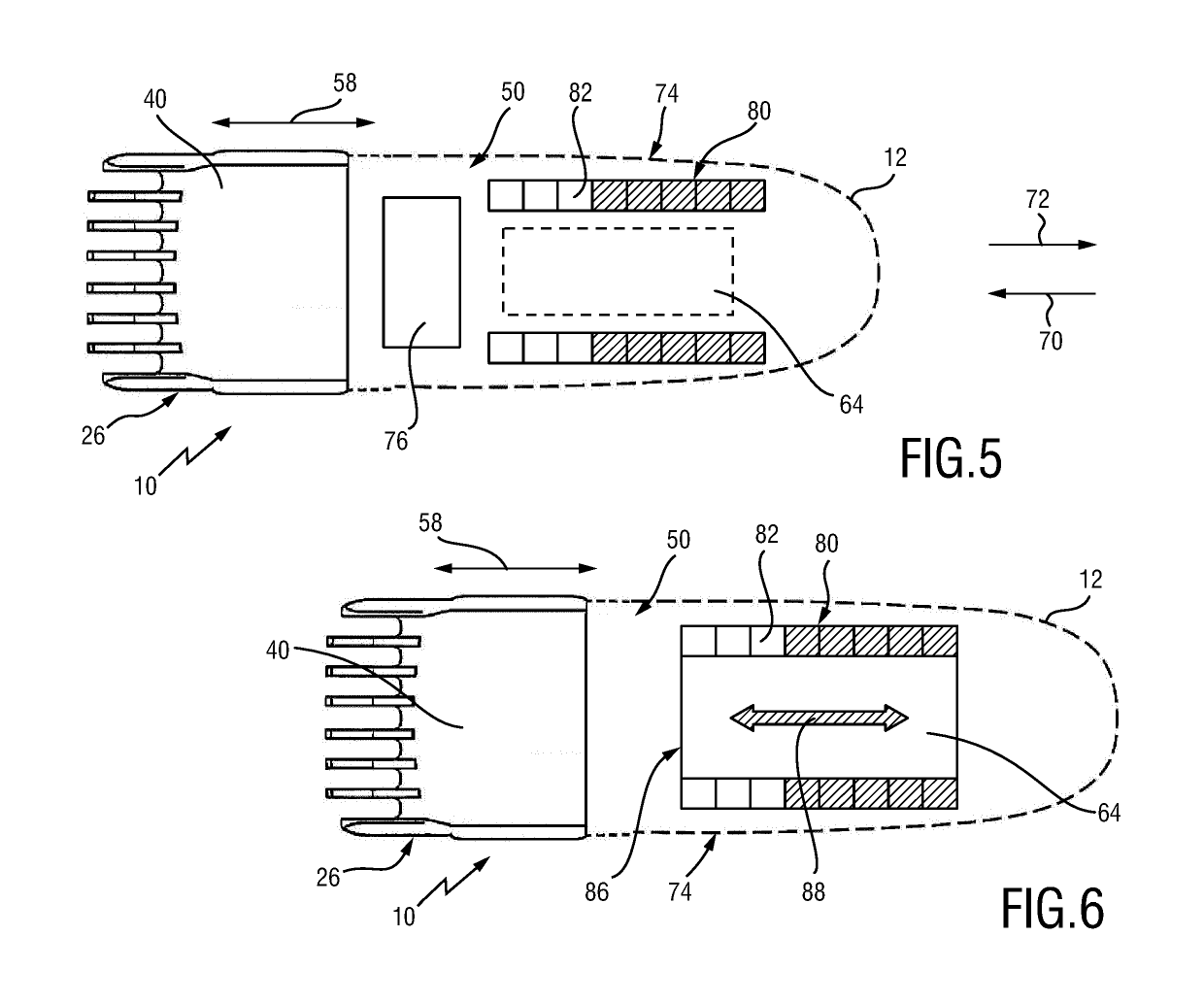
Adjustable spacing comb, adkustment drive and hair cutting appliance
The present disclosure relates to an adjustment drive (50) for an adjustable spacing comb (26) for a hair cutting appliance (10) and to a hair cutting appliance (10) that is fitted with an adjustable spacing comb (26). The present disclosure further relates to a method for operating an adjustable spacing comb (26) for a hair cutting appliance (10). The adjustment drive (50) comprises an actuator (52) that is configured for actuating a movable comb portion (40) of the adjustable spacing comb (26) with respect to a blade set (16) of the hair cutting appliance (10), and a proximity sensitive or touch sensitive sensor element (64), particularly a gesture control user input interface, wherein the sensor element (64) is configured to detect multi-faceted user inputs (70, 72) applied to the sensor element (64) and to output a user input signal that is derived from the multi-faceted user inputs (70, 72), and wherein the actuator (52) is operated on the basis of the user input signal.
Owner:KONINKLJIJKE PHILIPS NV
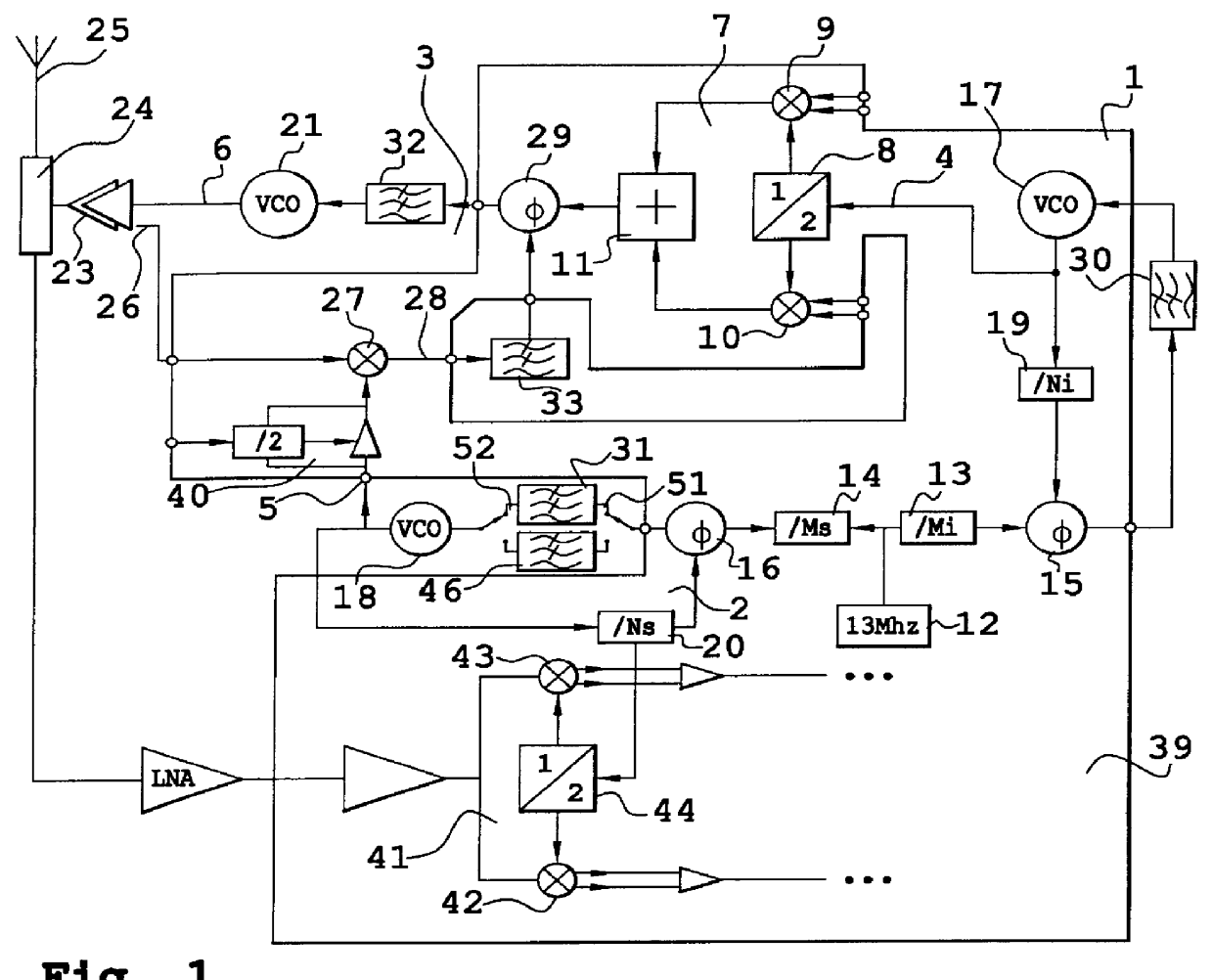
Transmit-receive system and transmission method, in particular for a mobile telephone
InactiveUS6161000ALow costImprove reliabilityResonant long antennasPulse automatic controlMobile phonePhase-locked loop
A transmit-receive system includes a phase-locked loop and a translation frequency loop. To bring about frequency hops, the frequencies in the two loops are varied with large increments in opposite directions. It is shown that the noise caused by the frequency division in the loops is thereby reduced.
Owner:DRNC HLDG INC
Method of initializing bolt pretension in a finite element analysis
ActiveUS20100076739A1Small incrementAvoid the needAnalogue computers for vehiclesDesign optimisation/simulationElement analysisMechanical engineering
In one aspect of the invention, each bolt is modeled using a beam element in a FEA model. To apply desired pretension to one or more bolts, at least one pretension-versus-time curve is specified. Each pretension-versus-time curve includes ramp portion, desired pretension portion and optional unloading portion. Duration_of the pretension-versus-time curve generally covers first 0.5-1% of total simulation time of a car crashworthiness analysis. Ramp portion starts from zero to desired pretension in a substantially linear manner, and hence being configured for applying desired pretension to a bolt gradually with smaller increments. Desired pretension portion is configured for ensuring the desired pretension can actually be applied to the beam element during an initialization process—a series of quasi-static analyses. Since the method is independent of the deformation of the beam, the method completely avoids the need to iteratively determine an axial strain or displacement that gives the desired pretension.
Owner:ANSYS
Clock data recovery system
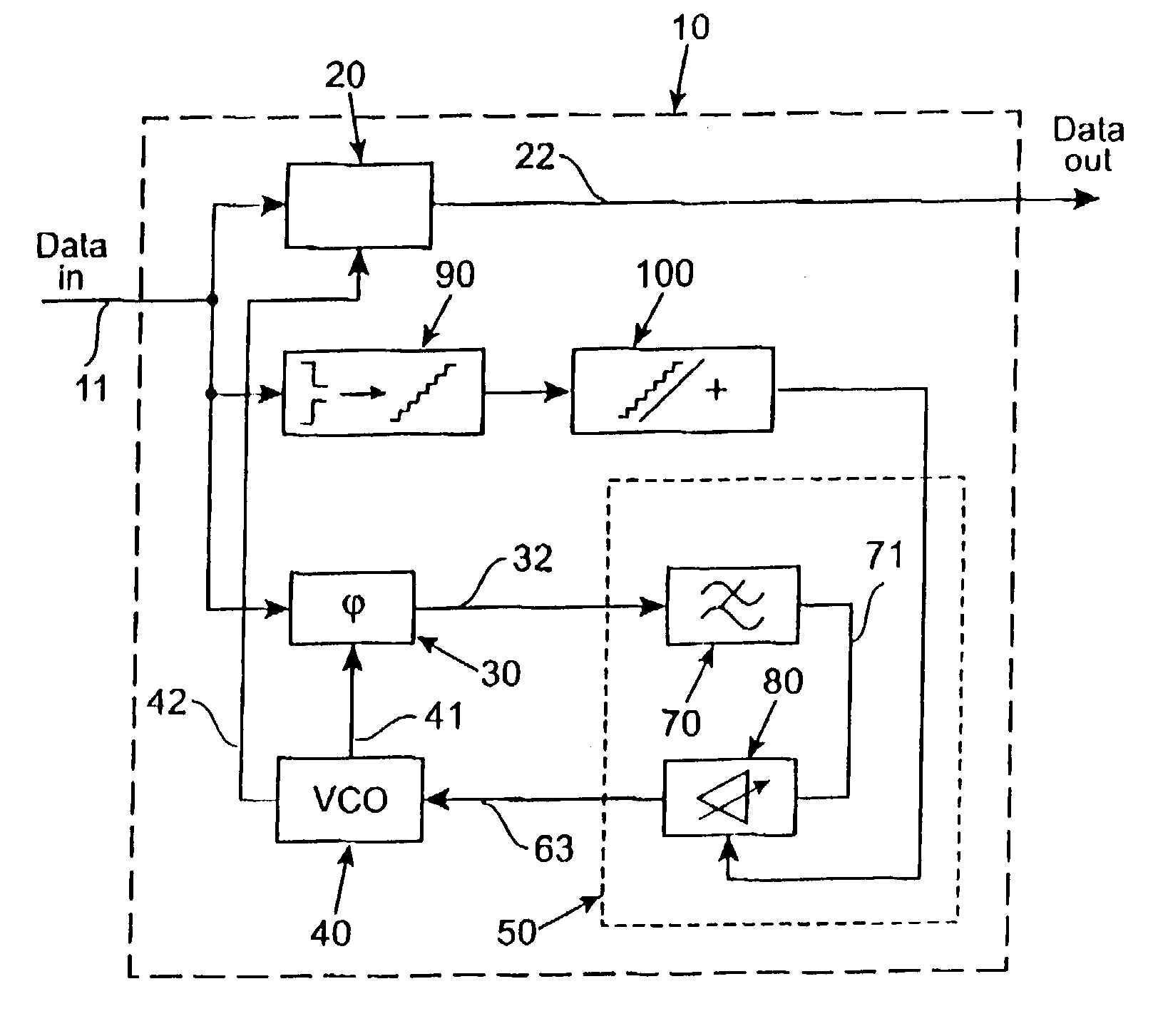
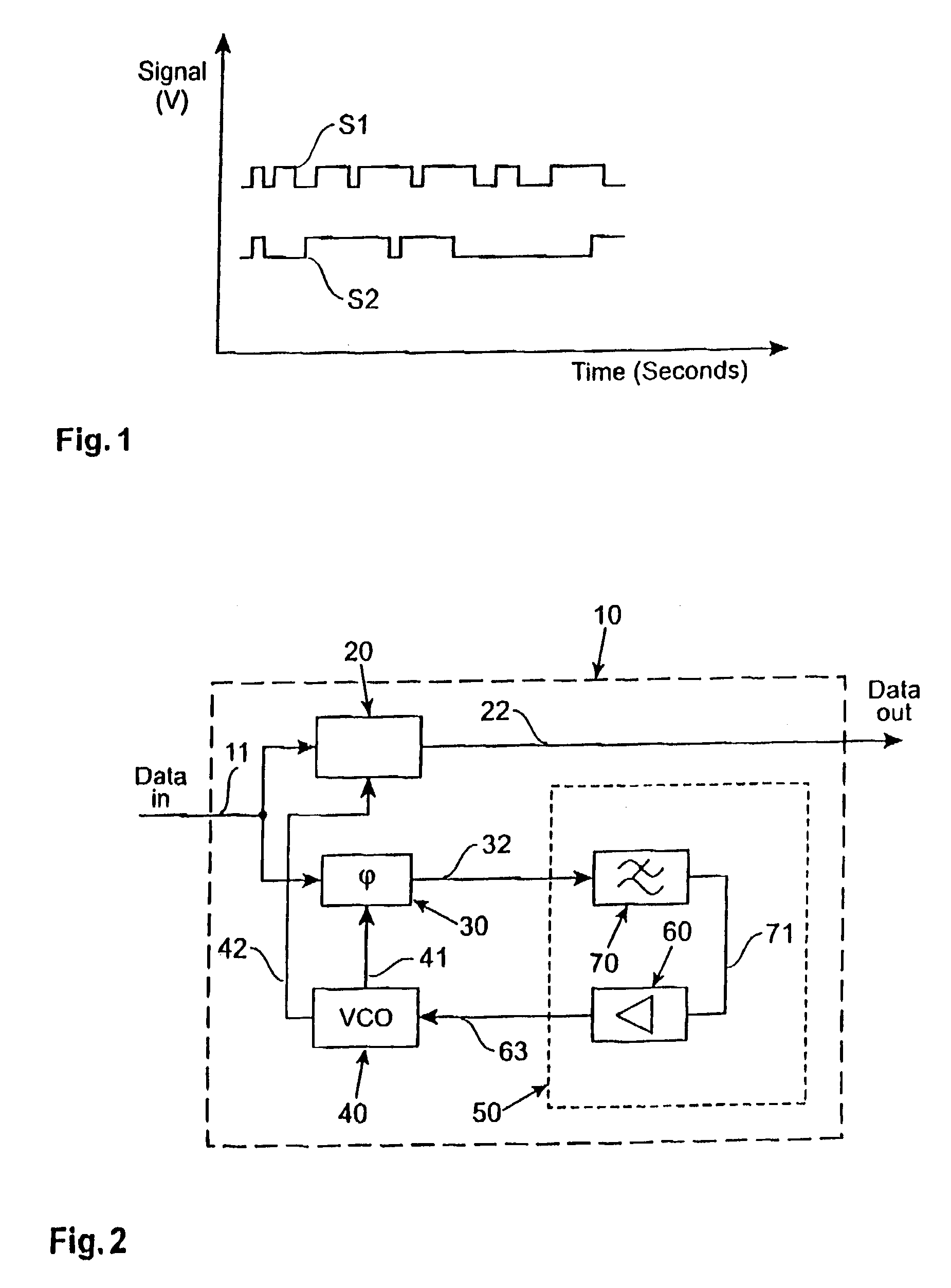
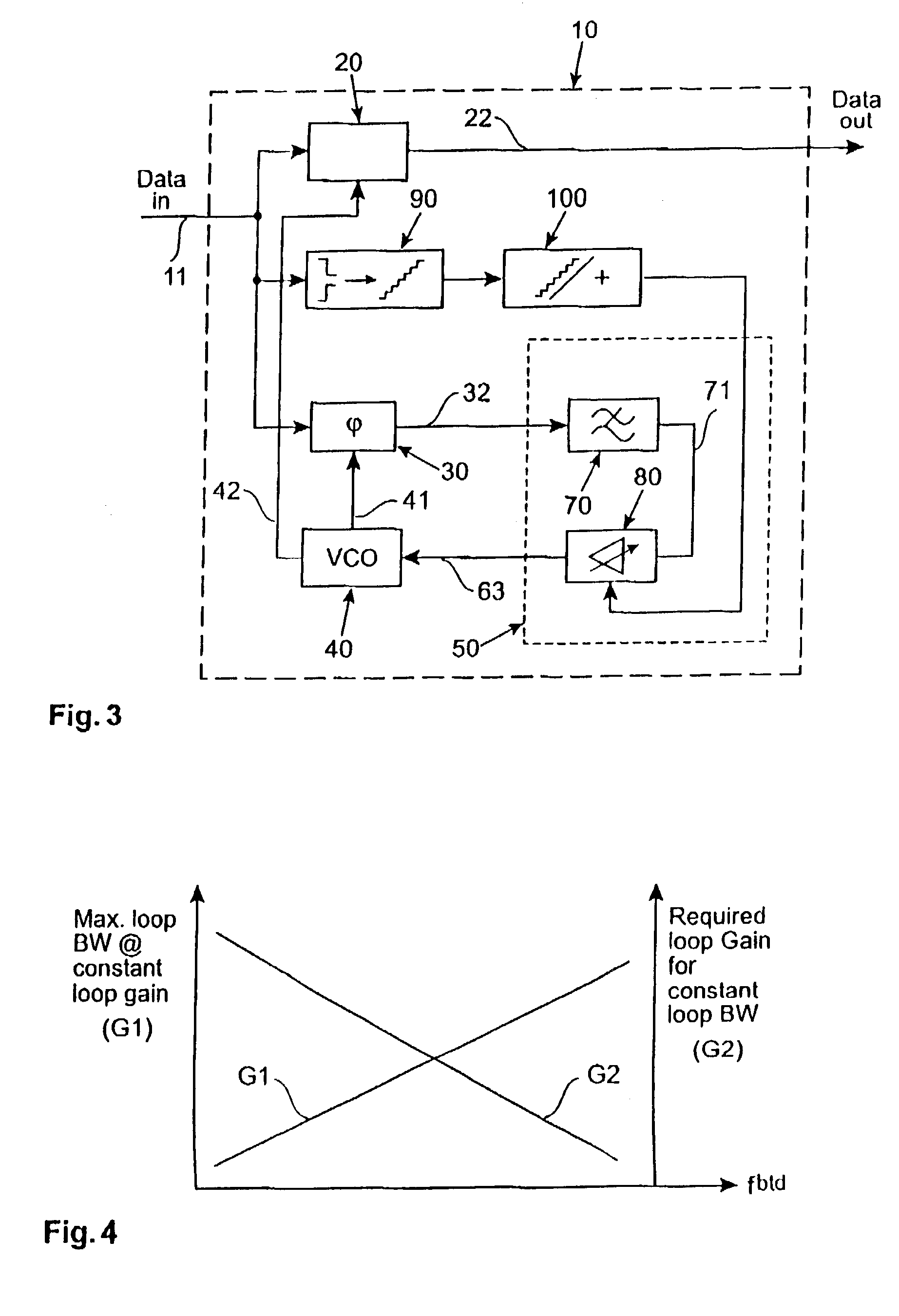
ActiveUS6981168B2Increase fashionIncrease loop bandwidthError preventionModulated-carrier systemsPhase detectorControl signal
A clock data recovery system is provided for resampling a clock signal according to an incoming data signal stream. It comprises a clock generator for generating said clock signal wherein one of the frequency and phase of that clock signal is dependent upon a control signal. It is further provided a phase detector operable to detect the phase difference between said clock signal and said incoming data signal stream and is operable to generate a phase difference signal. A loop controller has a variable-gain and is operable to control said clock generator by generating said control signal. That control signal is dependent in said phase difference signal and that variable-gain. The variable-gain is dependent upon a transition rate of the incoming data signal stream. The loop controller can comprise a low-pass filter to generate from the phase difference signal a low-pass filered phase signal and to adjust the bandwidth of the clock data recovery system. The loop controller further can comprise a variable-gain element to amplify the filtered signal in accordance with a received bit transition rate provided by a bit transition detector and a density calculator.
Owner:LENOVO GLOBAL TECH INT LTD
Hose holder
Owner:VOTYPKA EDWARD A
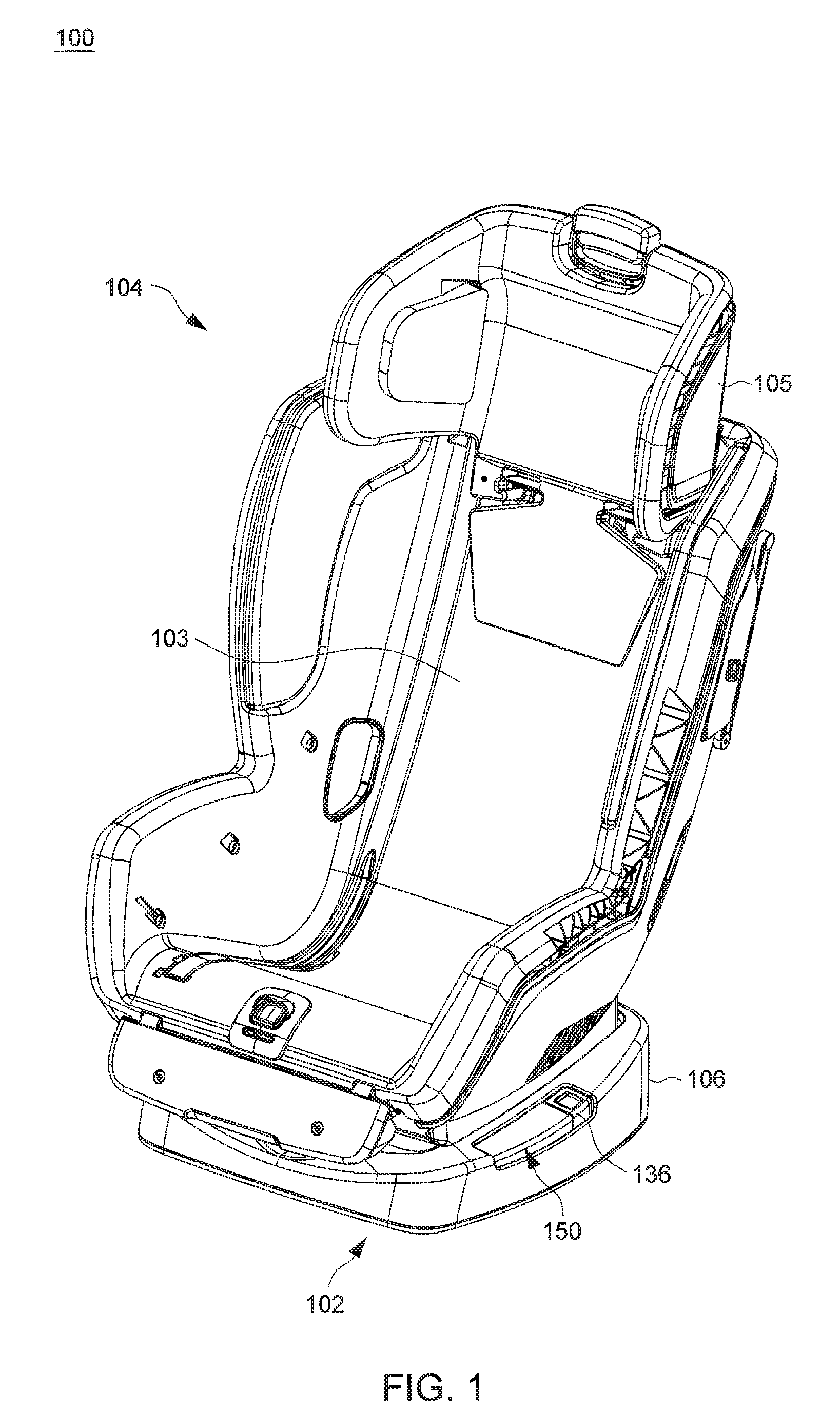
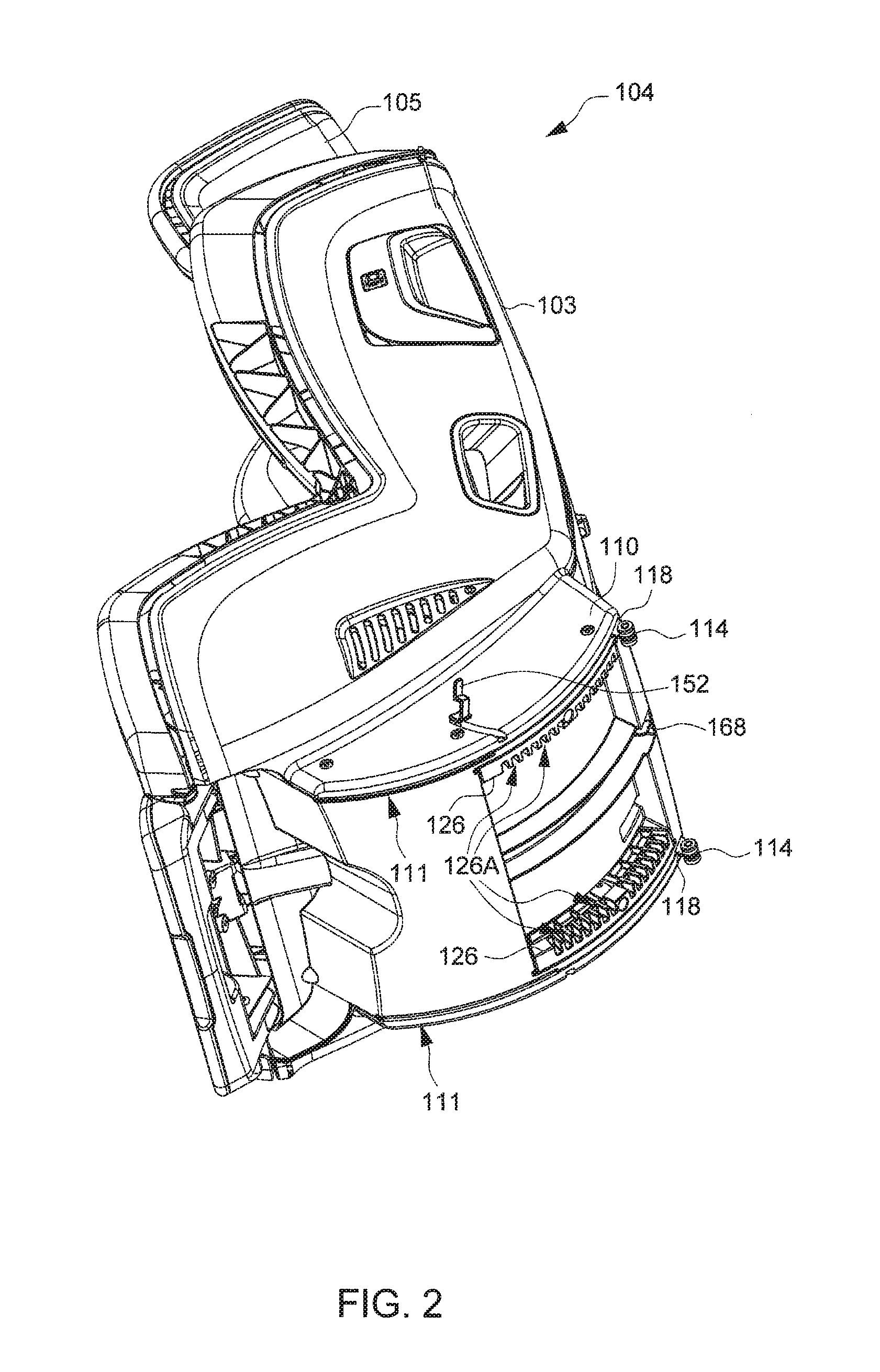
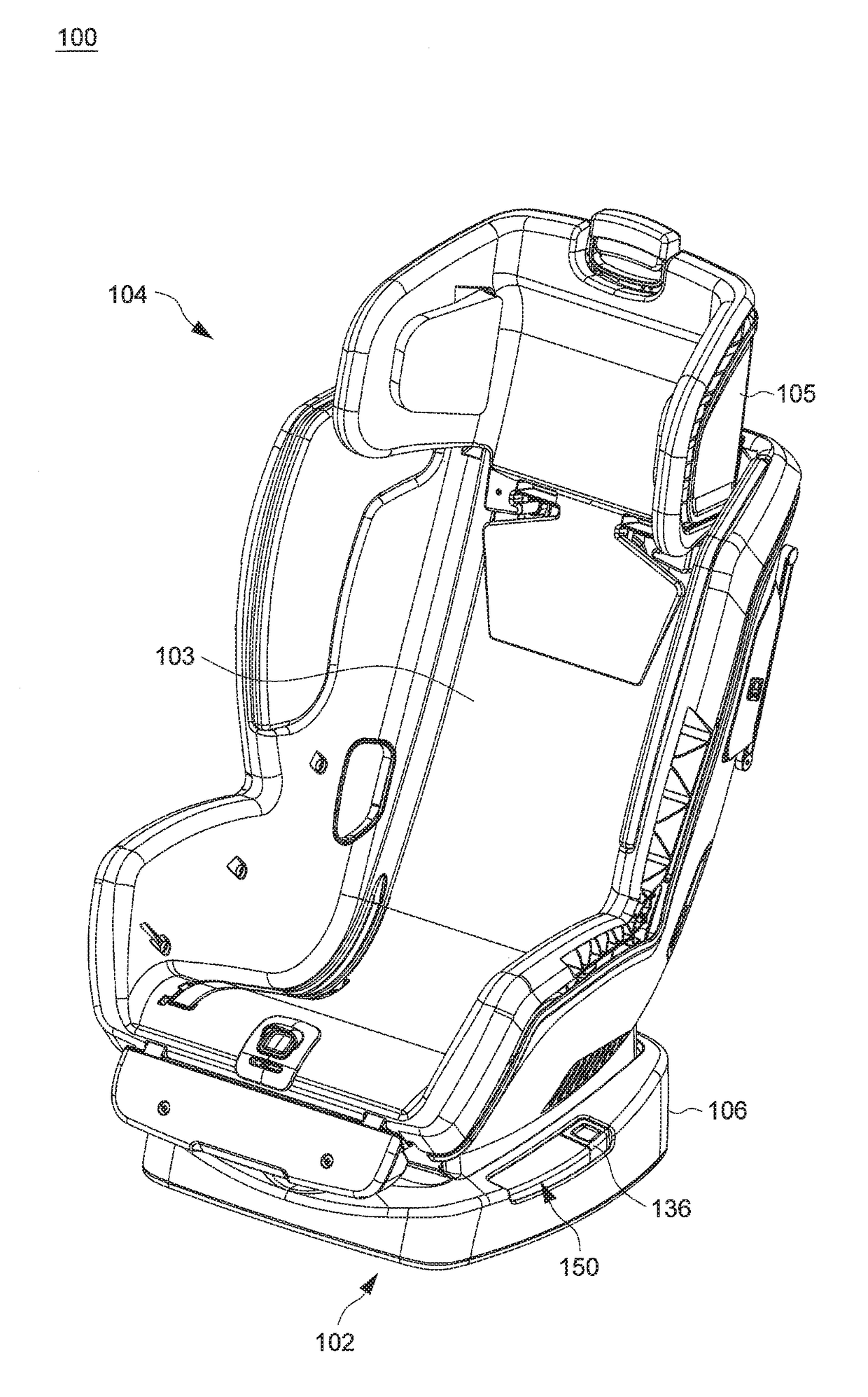
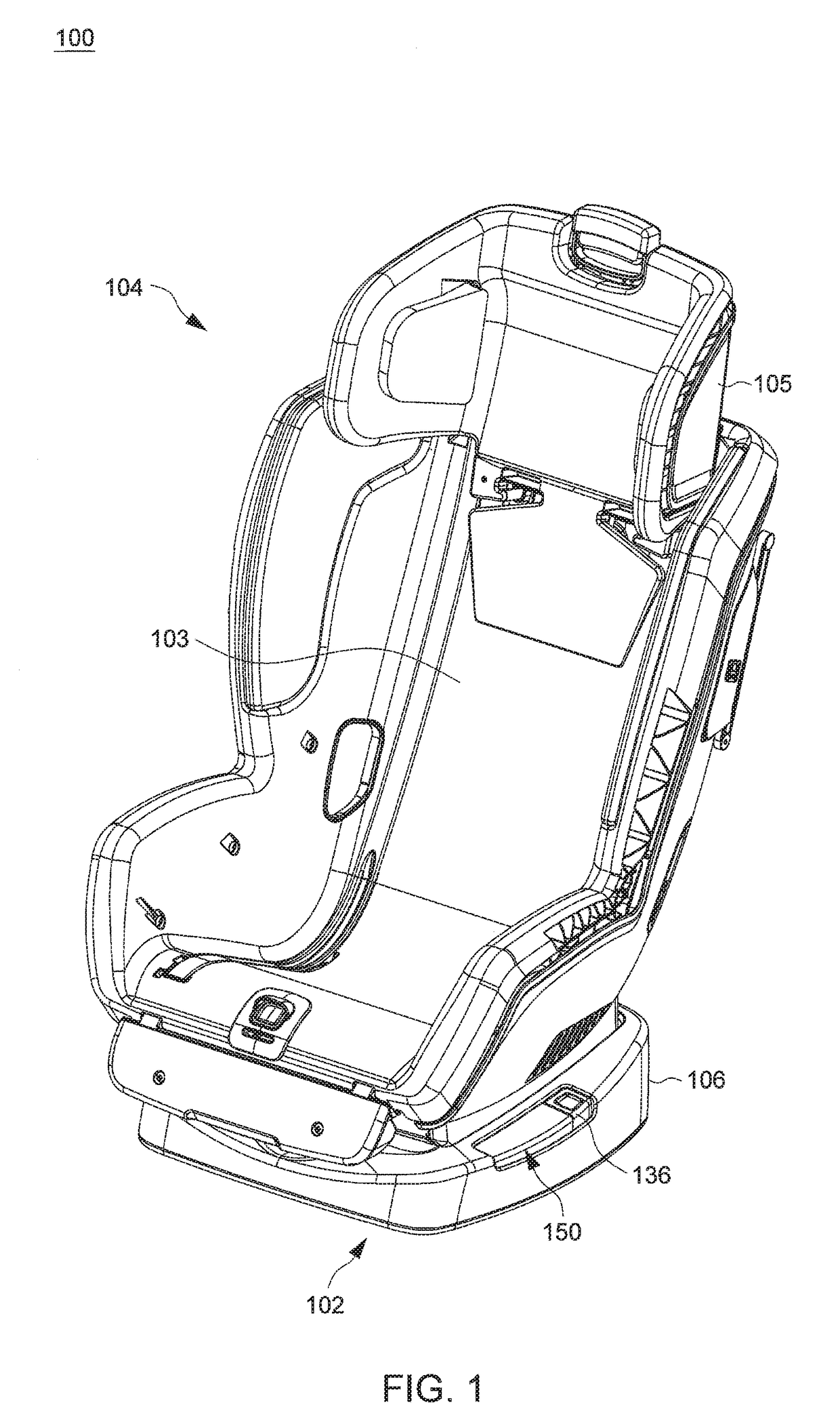
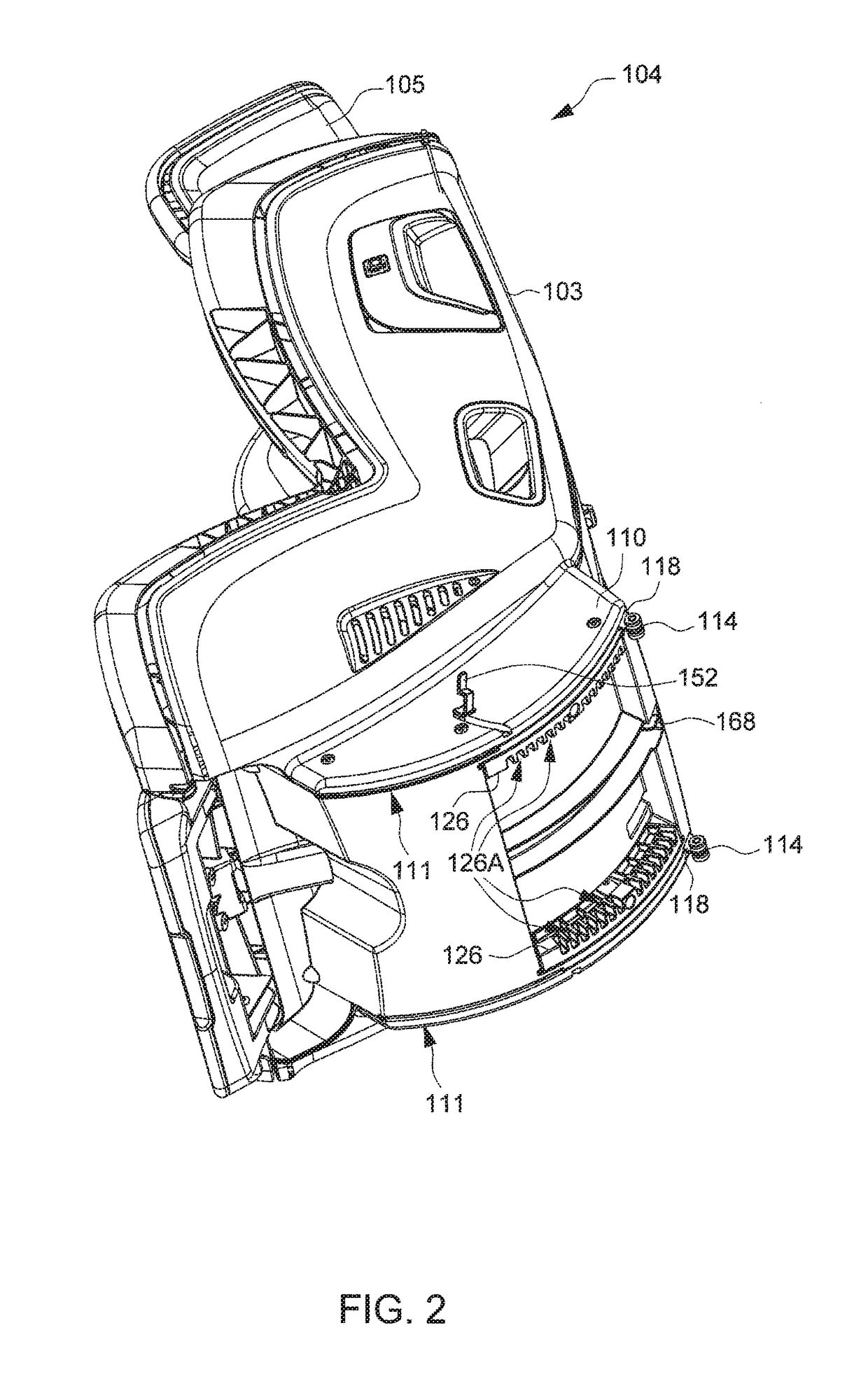
Child Safety Seat
ActiveUS20160176320A1Easy to operateEasy to adjustKids chairsChild seatsLocking mechanismMechanical engineering
A child safety seat includes a base having a shell body, a seat shell assembled with the base, the seat shell being adjustable between a plurality of recline positions relative to the base, and a lock mechanism operable to lock the seat shell with the base at any of the recline positions. The lock mechanism includes two latches assembled with the shell body and movable to engage with and disengage from the seat shell, and two release buttons respectively disposed at a left and a right side of the base and respectively coupled with the two latches, each of the two release buttons being independently operable to drive concurrent unlocking displacements of the two latches.
Owner:WONDERLAND SWITZERLAND AG
Webpage link protection method based on control character coding and steganography
InactiveCN104050400AStop the spreadReduce negative impactProgram/content distribution protectionControl characterWeb page
The invention relates to a webpage link protection method based on control character coding and steganography. Watermark information can be embedded through selected control characters which are not displayed at a browser end, and thus link information in a webpage can be protected. First, the webpage is partitioned into blocks according to links, watermark information with any bit is generated according to all the characters of all the sub-blocks and coded into corresponding control characters to be embedded into the webpage, and a webpage with watermarks is generated; in detection, whether the link blocks are tampered or not is judged by comparing the relations between different bit numbers and thresholds of extracted watermarks and reconstructed watermarks corresponding to the link blocks; when tampered link blocks are detected, all attribute information including link addresses is deleted, and blocks are marked with warning mark information, so that a prompt is given to a user. Through the webpage link protection method based on control character coding and steganography, link block information in the webpage can be effectively protected; when the link blocks are tampered, the tampering can be detected out in time, and false information is prevented from being propagated, so that negative effects of tampered link blocks on images ad business of enterprise and public institutions are reduced.
Owner:SOUTHWEST JIAOTONG UNIV
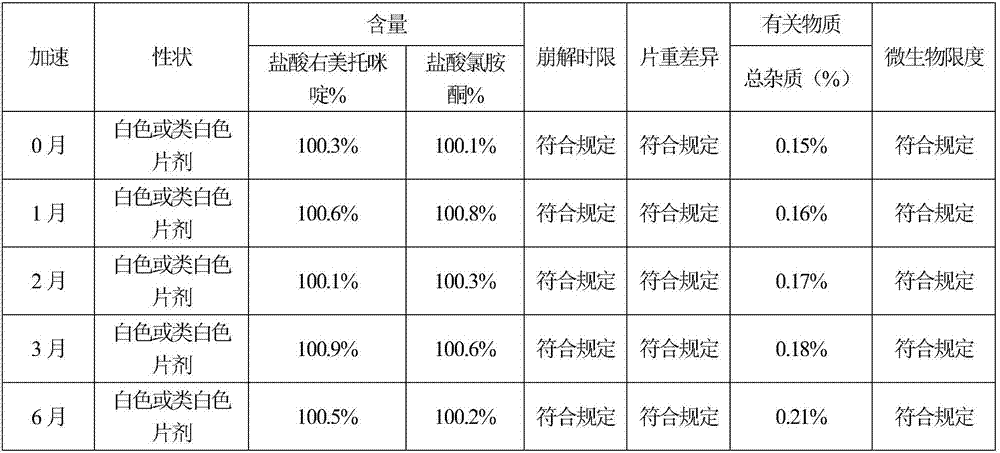
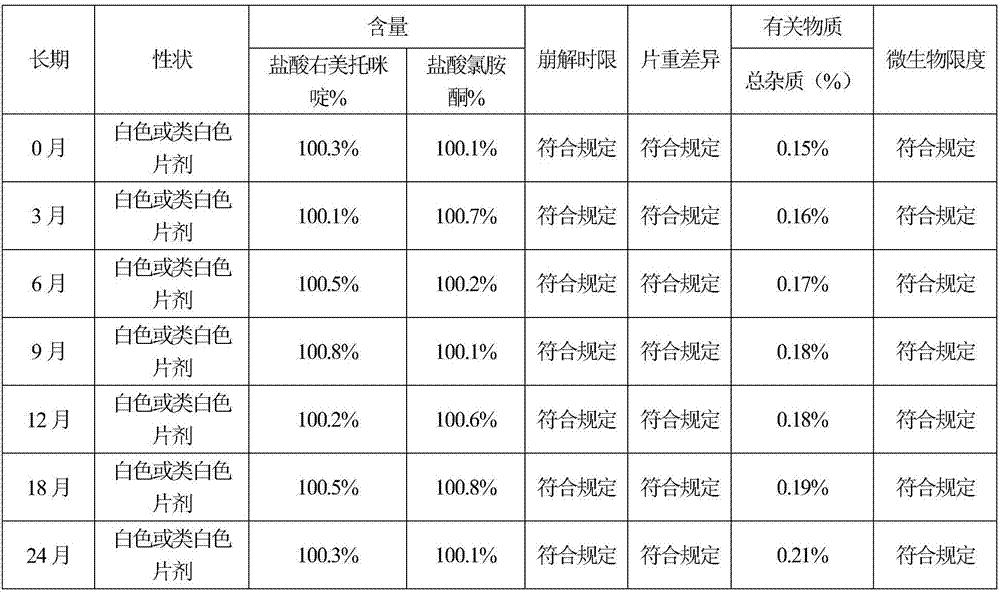
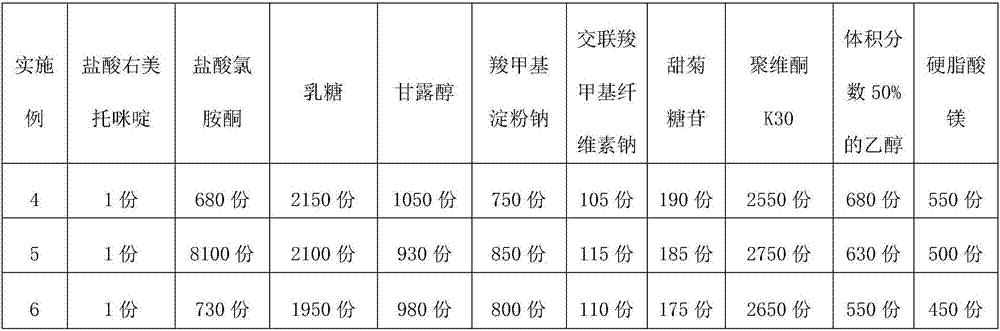
Sublingual tablet for anaesthesia and preparation method thereof
InactiveCN107137399AAvoid first pass effectDisintegrates quicklyOrganic active ingredientsAnaestheticsAdhesiveHepatic first pass effect
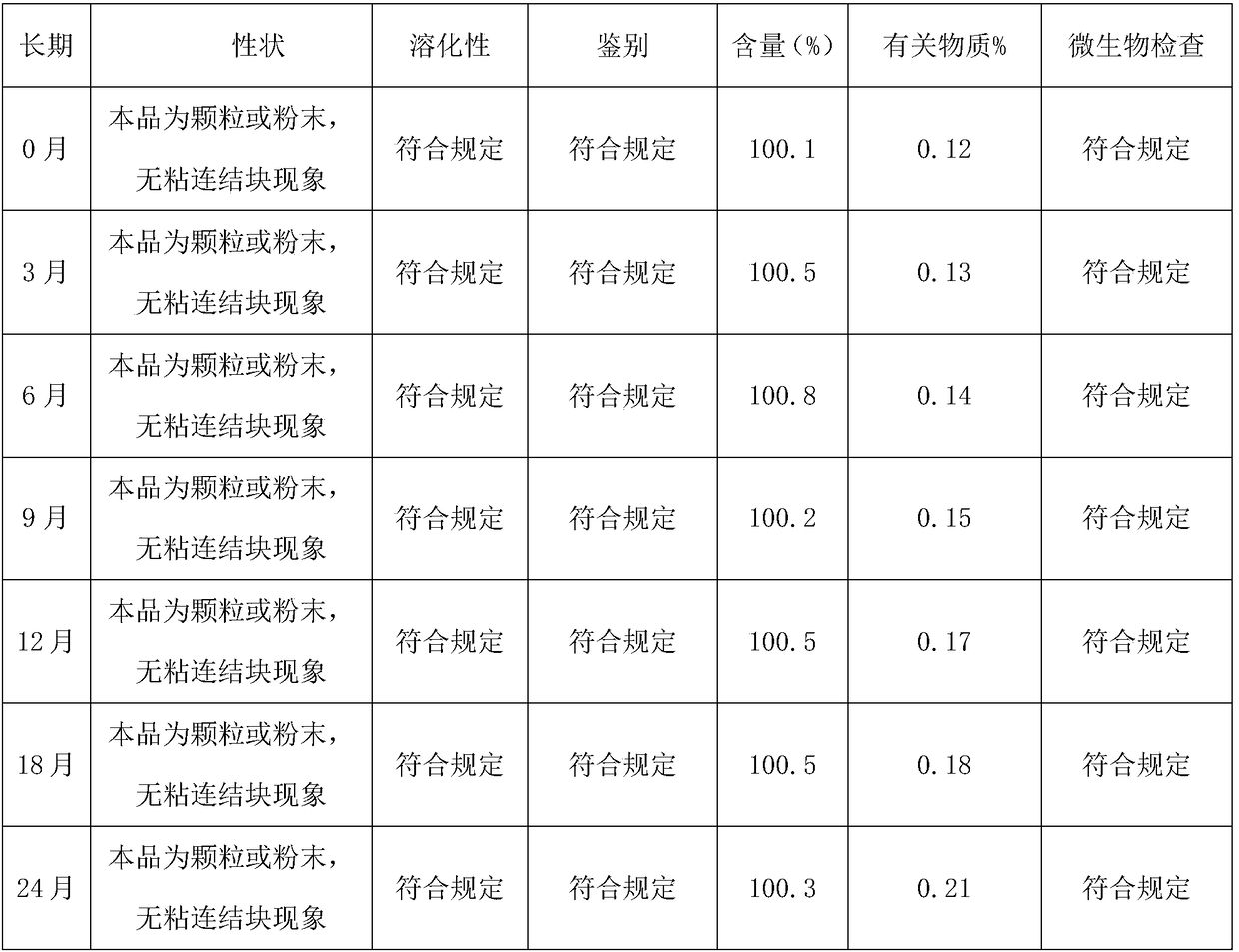
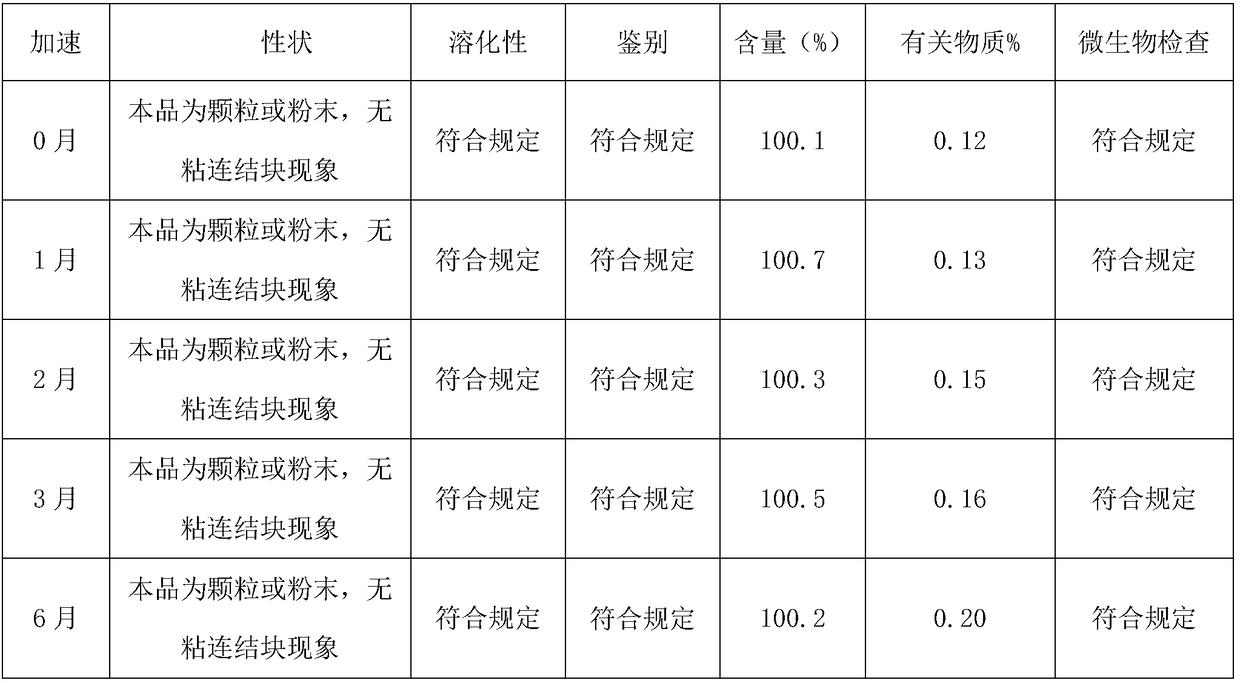
The invention discloses a sublingual tablet for anaesthesia. The preparation method of the sublingual tablet comprises the following steps: taking dexmedetomidinehydrochloride and ketamine hydrochloride as raw materials, adding a certain amount of a filling agent, a disintegrating agent, a corrigent, an adhesive and a lubricant, and performing pretreatment, mixing, granulatinganddrying, total blending, aluminum plastic inner packaging, outer packaging and the likeseparately. The sublingual tablet does not require building a special administrationchannel, is directly dosed through sublingual veins, avoids the liver first pass effect, takes effect quickly and is good in effect; particle angles of the sublingual tablet are all smaller than 35 DEG C duringpreparation, so that the particle mobility of the sublingual tablet is good, finished products is rapid to disintegrate and can be completelydisintegrated within 3 minutes, the weight difference of finished products is small, the increment of impurities is small during storage, the stability is good, the shelf life reaches 24 months, the preparation technology is simple and practical, and the sublingual tablet is worthy of market promotion.
Owner:CHONGQING YUBEIHAI TECH CO LTD
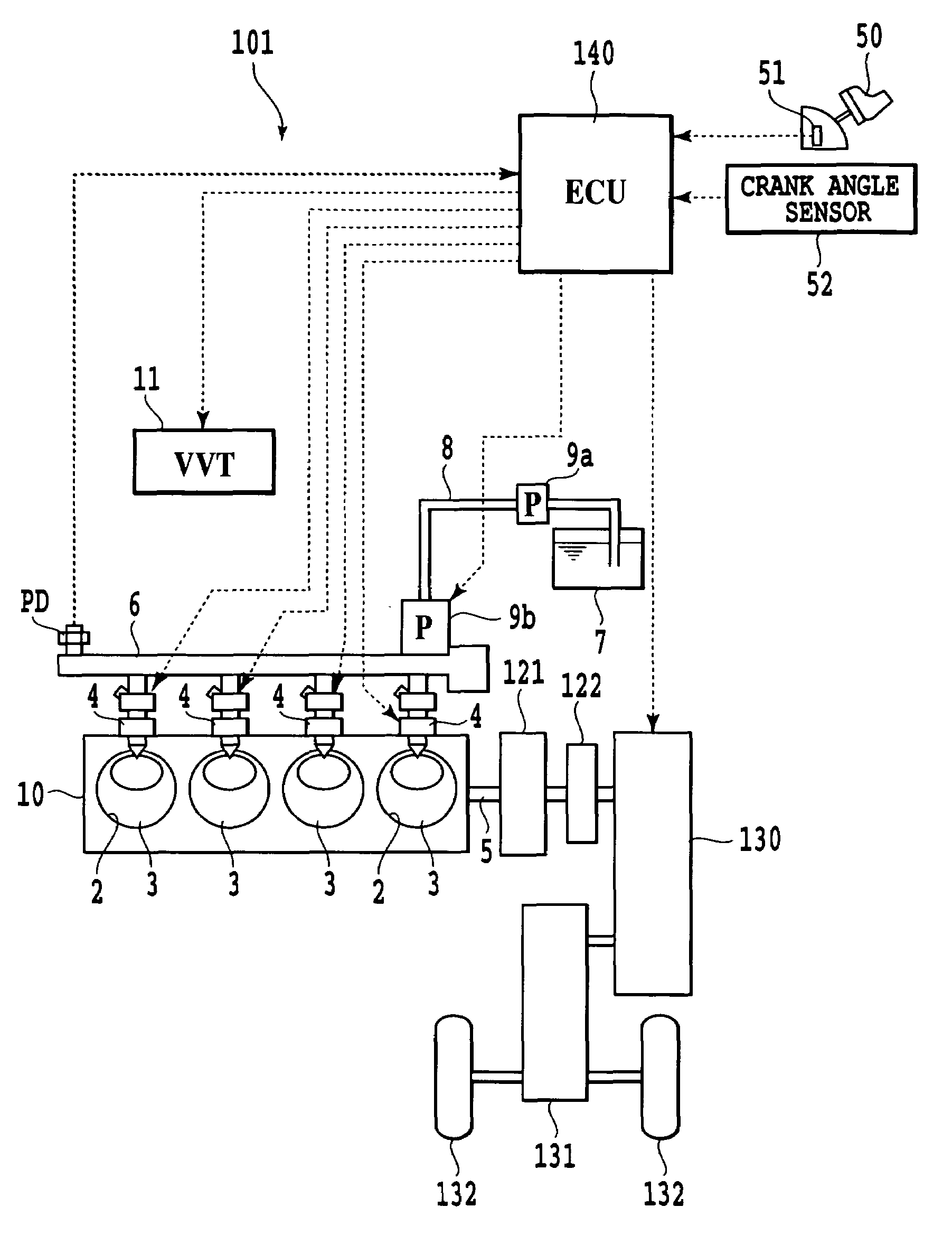
Device and method for controlling engine
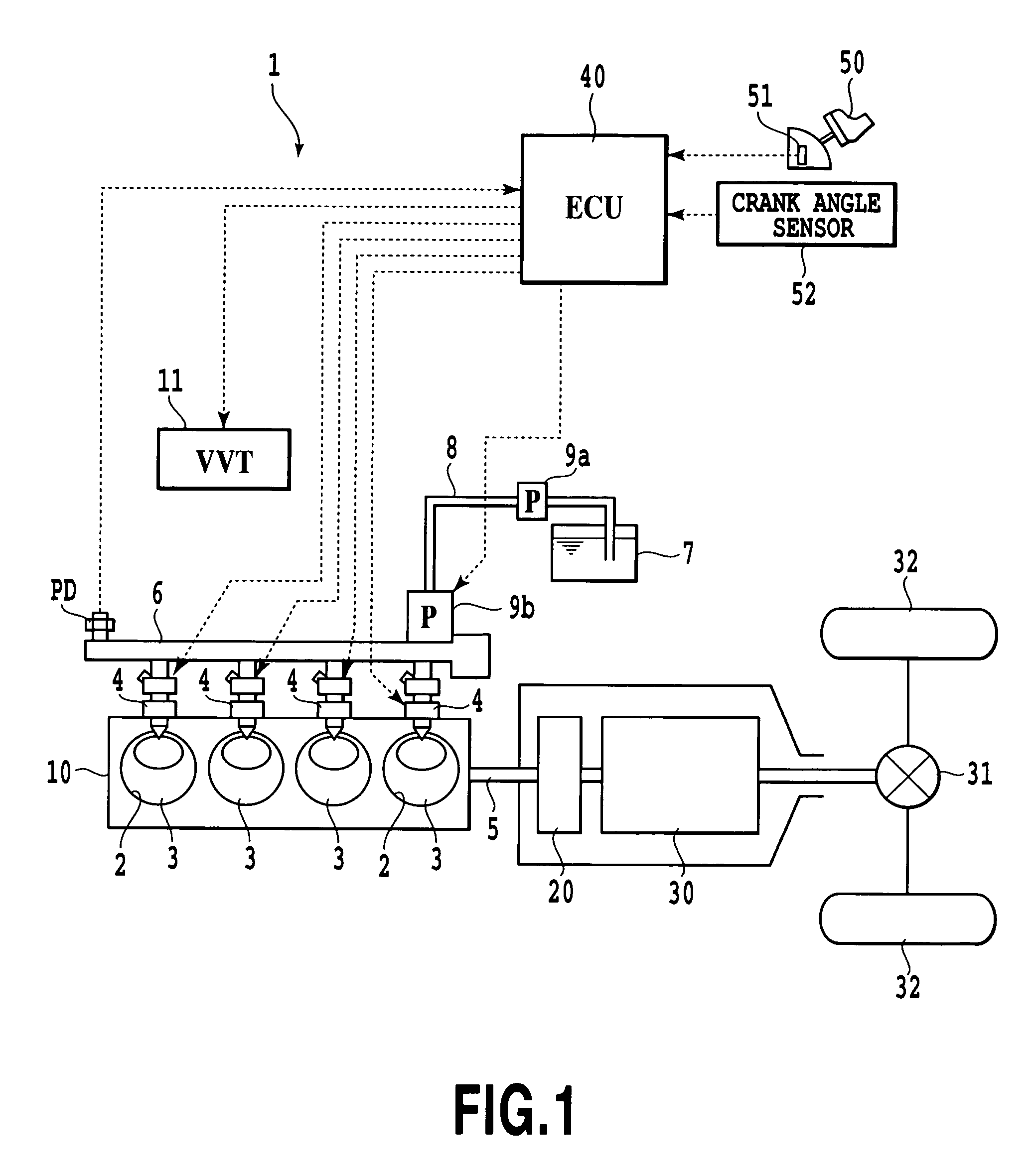
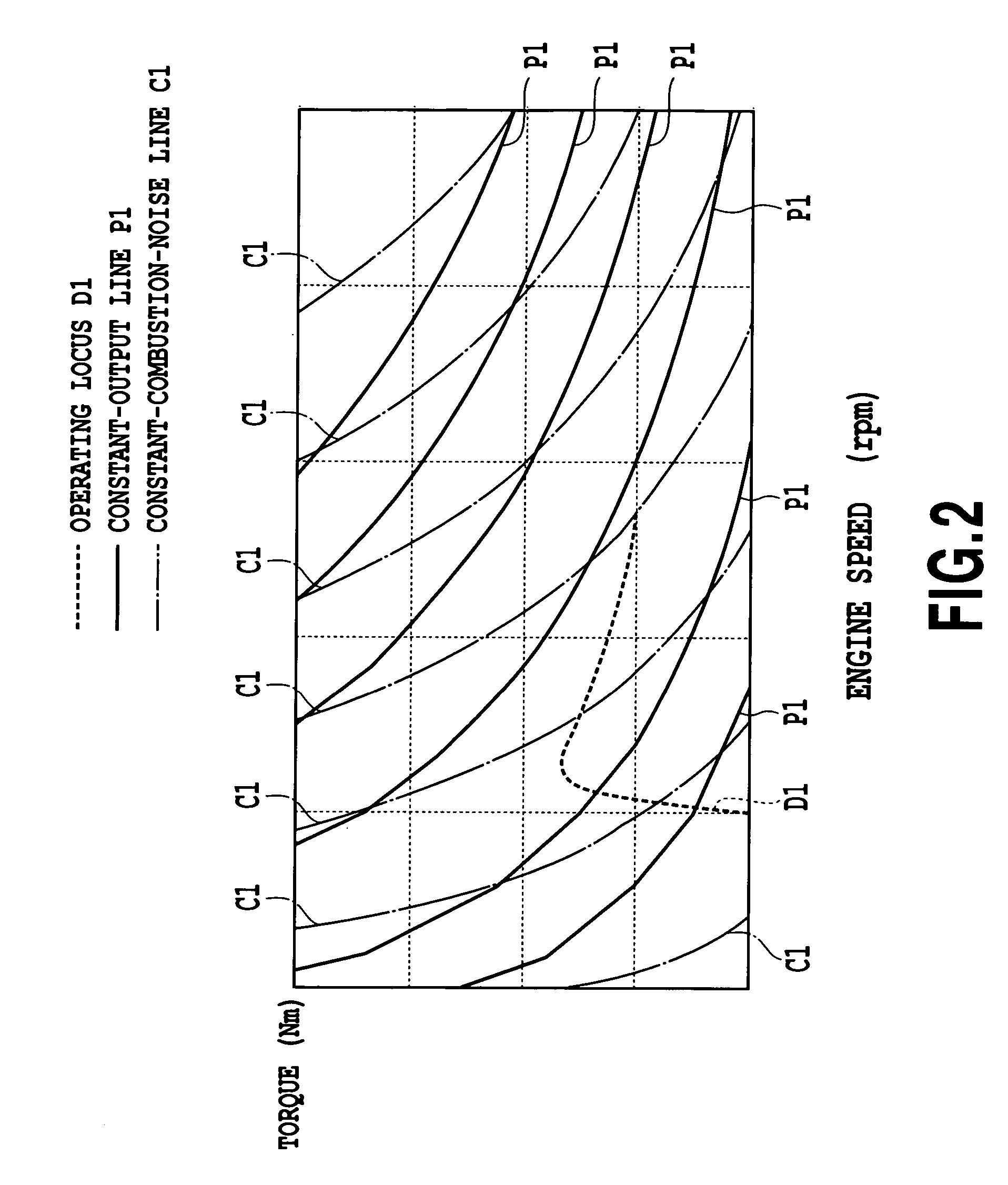
InactiveUS7337767B2Slow changeAvoid rapid changesAnalogue computers for vehiclesElectrical controlCombustion noiseCombustion chamber
In a device for controlling an engine, comprising combustion noise suppressor for suppressing combustion noise of a combustion chamber and controller for controlling the combustion noise suppressor, the device further includes control amount setter for setting a control amount of the combustion noise suppressor so that target combustion noise characteristics corresponding to a required amount of acceleration or deceleration exhibit a slower change in combustion noise than combustion noise characteristics corresponding to output characteristics of the engine over before and after acceleration or deceleration, wherein the controller controls the combustion noise suppressor according to the control amount set by the control amount setter. On the event of a rapid acceleration, a rapid change in the combustion noise in the initial stage of the acceleration is prevented to improve driving comfort.
Owner:TOYOTA JIDOSHA KK
Computer numerical control of fiber tension in fiber processing
ActiveUS8116899B1Speed up the processMuch-improved end-productsFilament handlingDrafting machinesNumerical controlFiber bundle
A CNC tensioning system for applying tension in one or more fibers or fiber bundles drawn into a fiber process in a fiber movement direction includes a reverse torque mechanism that applies a net torque on the one or more fibers or fiber bundles in a reverse direction from the fiber movement direction; a motion-control motor that drives the reverse torque mechanism to apply a net torque on the one or more fibers or fiber bundles in a reverse direction from the fiber movement direction; and a computer system controlling the motion-control motor to incrementally control net torque on the one or more fibers or fiber bundles in a reverse direction from the fiber movement direction in discrete incremental values.
Owner:EBERT COMPOSITES
Manufacturing technology method of titanium and titanium alloy bar wires used for manufacturing additional materials
PendingCN110735059AImprove quality levelStable ionizing arcAdditive manufacturing apparatusManufacturing technologyIngot casting
The invention provides a manufacturing technology method of titanium and titanium alloy bar wires used for manufacturing additional materials. The method comprises the following specific steps that needed raw materials are smelted, sponge titanium and an intermediate alloy are weighed, the raw materials are added into a mixing machine to be mixed uniformly, and obtained bars for EB smelting are subjected to ingot casting to obtain EB cast ingot blanks; the surfaces of the obtained EB cast ingot blanks are machined mechanically, the removing thickness of the milled surfaces is 2-5 mm, and surface defects are subjected to coping and polishing treatment; and bars and wires are obtained by means of one fire time and multiple fire times and metal pressure machining treatment, and among the different fire times, a straightening machine carries out online straightening, and a centerless lathe carries out scaling and polishing. The manufacturing technology is simple and low in cost, the obtained bar wires contain low content of impurity elements such as O and N, the component is more pure, and the comprehensive performance of additional material manufacturing parts is effectively improved.
Owner:LUOYANG SUNRUI TI PRECISION CASTING
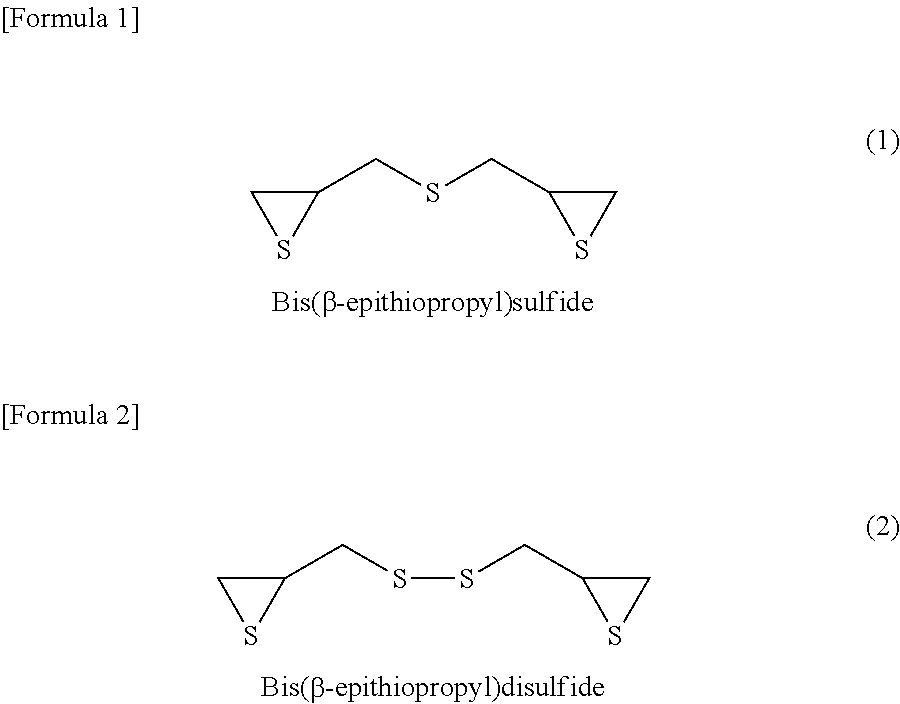
Method for producing composition for optical material
ActiveUS20140378628A1High refractive indexHigh strengthOptical partsOptical elementsInorganic compoundRefractive index
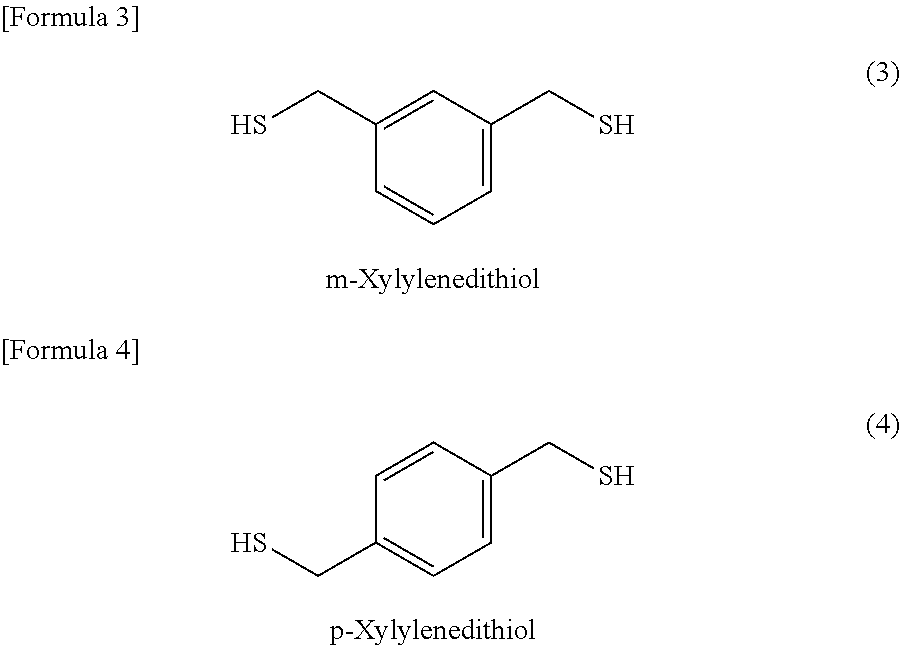
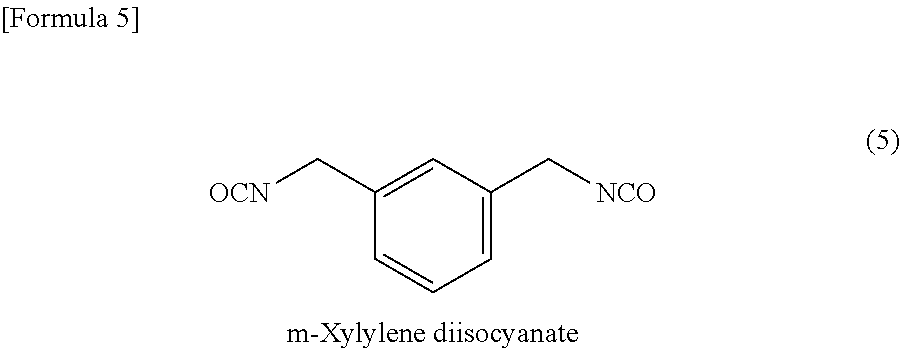
According to the present invention, a composition for optical material, which is capable of providing a homogeneous optical material, can be prepared through pre-polymerization reaction between (a) an inorganic compound having a sulfur atom and (b) an episulfide compound using a hindered amine as a catalyst, followed by mixing with (c) a polythiol compound and (d) a polyisocyanate compound. Moreover, this composition for optical material can be polymerized and cured to thereby provide an optical material having high refractive index (ne of 1.73 or higher), high strength (an elongation of 13% or more in three-point bend test and good drilling resistance), and high heat resistance (a softening point of 70° C. or higher, as measured by TMA).
Owner:MITSUBISHI GAS CHEM CO INC
Adjustable spacing comb, adjustment drive and hair cutting appliance
An adjustment drive is provided for an adjustable spacing comb for a hair cutting appliance. The adjustment drive includes an actuator, a sensor element and a control unit. The actuator actuates a movable comb portion of the adjustable spacing comb with respect to a blade set of the hair cutting appliance. The sensor element detects multi-faceted user inputs applied to the sensor element by a user and to output a user input signal that is derived from the multi-faceted user inputs. The actuator is operated on the basis of the user input signal. The control unit is coupled to the actuator and to the sensor element, and converts the user input signal into an actuator operating signal. The multi-faceted user inputs include detected user input speed of an input stroke by the user.
Owner:KONINKLJIJKE PHILIPS NV
Rectal suppository for anaesthesia and preparation method thereof
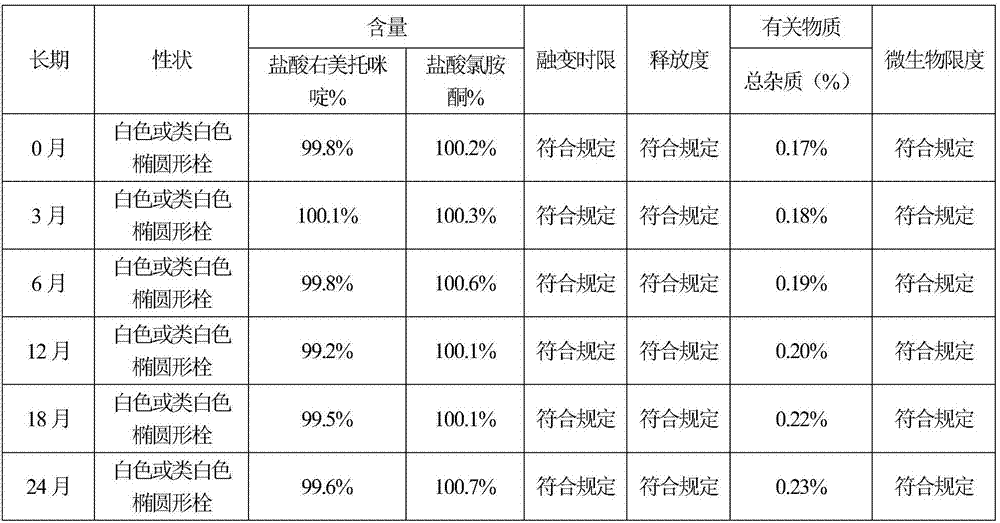
InactiveCN107137398AAvoid first pass effectSmall incrementOrganic active ingredientsSuppositories deliveryGynecologyRectocutaneous
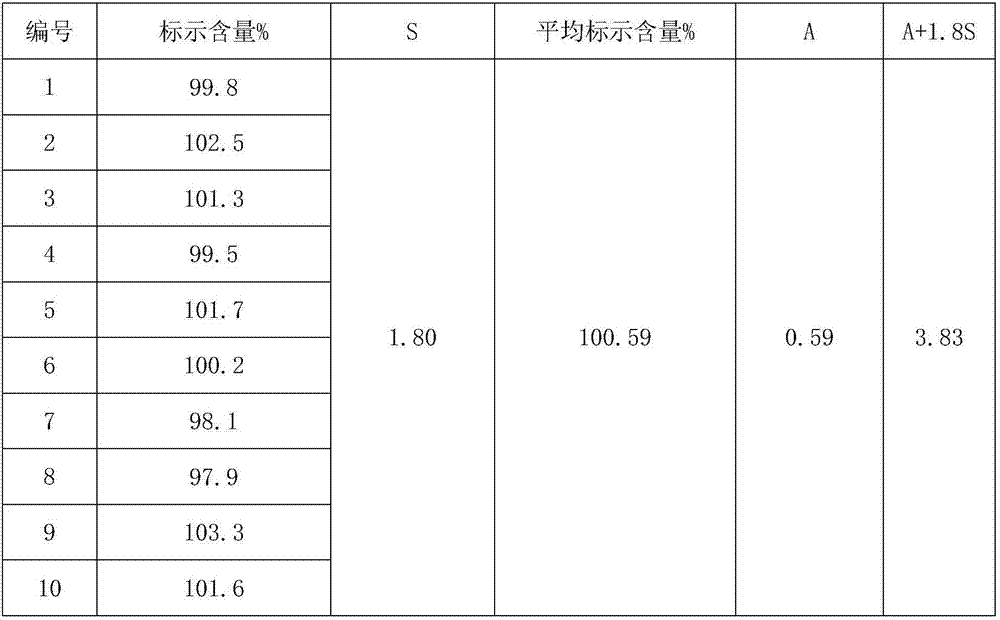
The invention discloses a rectal suppository for anaesthesia. The preparation method of the rectal suppository comprises the following steps: taking dexmedetomidinehydrochloride and ketamine hydrochloride as raw materials, adding a certain amount of matrix, a surfactant, a diluent, a humectant and a bacteriostat, and performing matrix melting, mixing, membrane injection, cooling shaping and packagingseparately. The rectal suppository does not require building a special administrationchannel, is directly dosed through arectum, avoids the liver first pass effect, takes effect quickly and is good in effect; the melting temperature of the rectal suppository is between 32EDG C to 35 EDG C, not only is beneficial to product storage, but also does not affect melting of the rectal suppository during use, meanwhile, the content of the rectal suppository is uniform and stable, A plus 1.8S of multiple products is smaller than 8, the product stability is good, the shelf life reaches 24 months, the increment of impurities is small during storage, the preparation process is simple and practical, and the rectal suppository is worthy of market promotion.
Owner:CHONGQING YUBEIHAI TECH CO LTD
Preparation method and application of Cs5SiFe (OH2) W11O39. 6H2O sustained and controlled release capsules
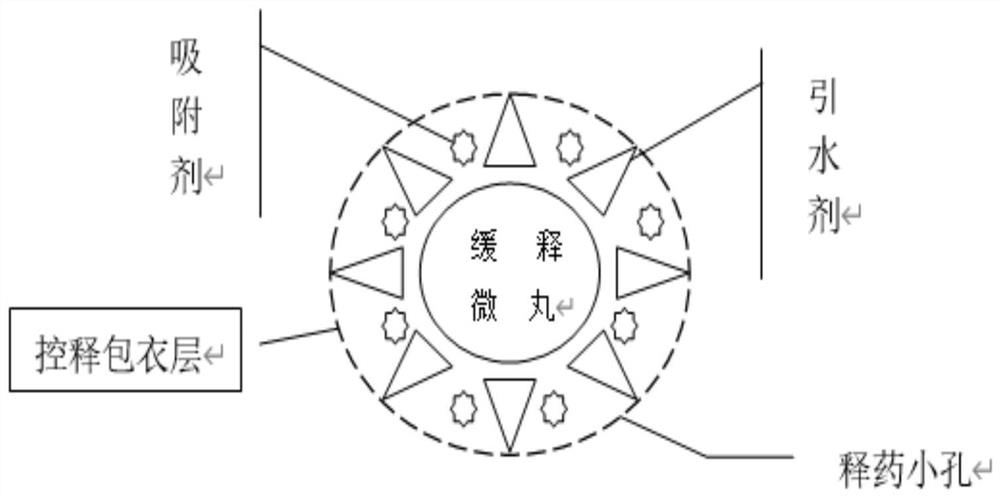
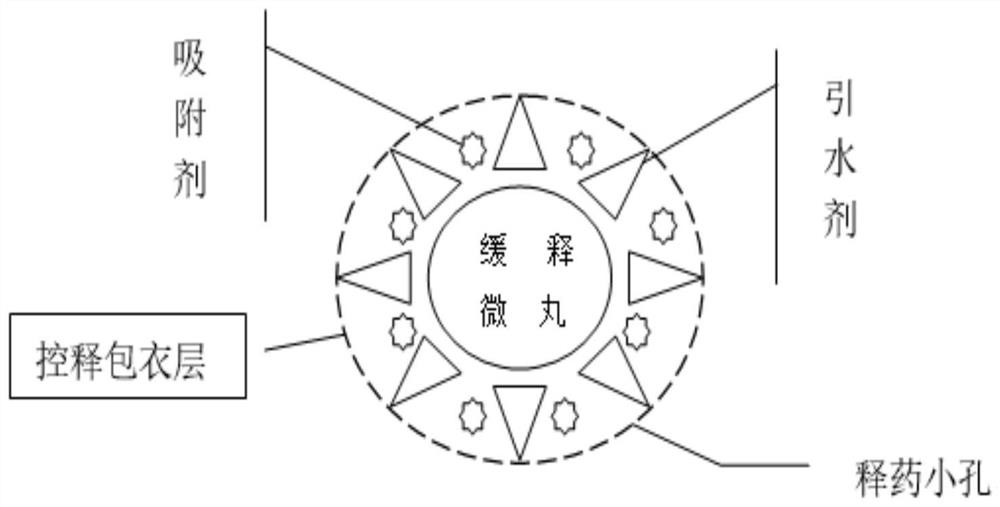
ActiveCN111904949AUniform release in vitroImprove anti-tumor activityHeavy metal active ingredientsInorganic non-active ingredientsSustained release pelletsSodium bisulfate
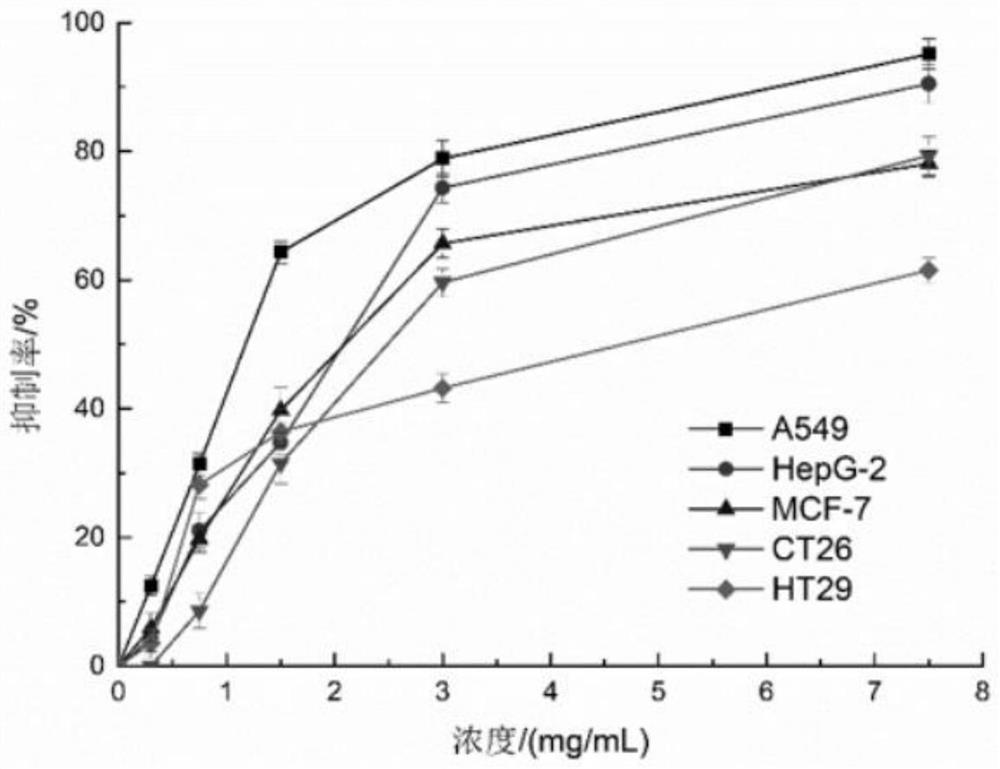
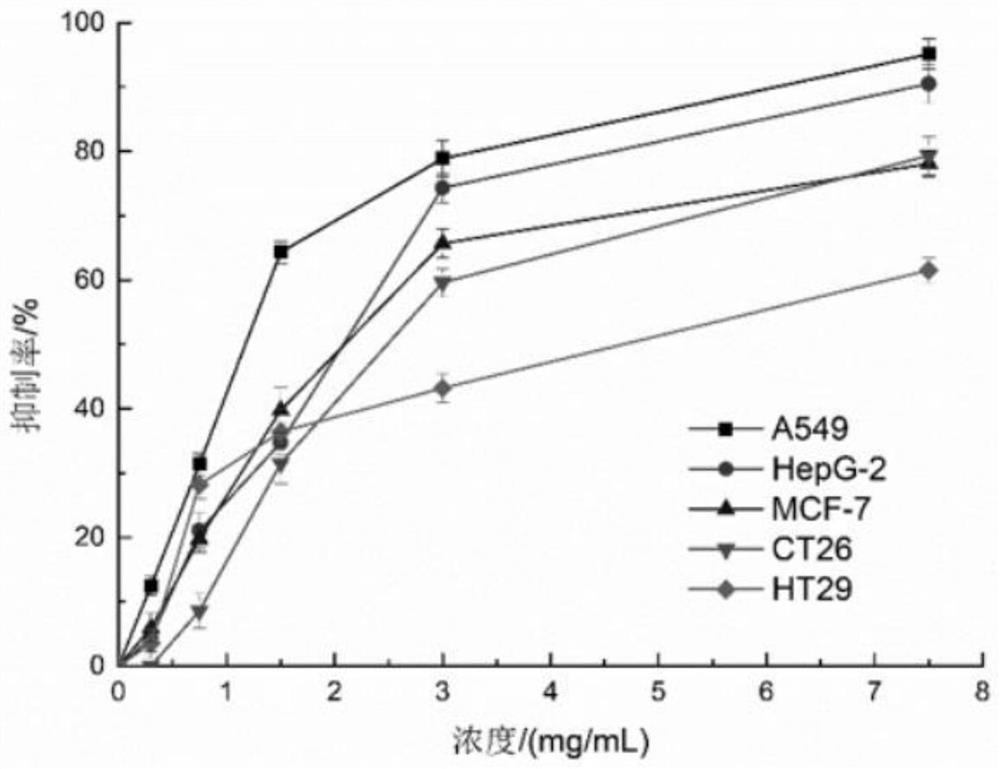
The invention relates to a preparation method of Cs5SiFe (OH2) W11O39. 6H2O sustained and controlled release capsules. The preparation method comprises the following steps of by taking Cs5SiFe (OH2) W11O39. 6H2O as a raw material and lactose, ethylcellulose, hydroxypropyl methylcellulose, silicon dioxide, montmorillonite, sodium chloride, cellulose acetate, dibutyl phthalate, dimethicone, magnesium stearate, sodium bisulfite and lauryl sodium sulfate as auxiliary materials, respectively preparing sustained-release pellets, performing wrapping with an adsorption water diversion layer, performing wrapping with a controlled-release coating layer, and finally, performing capsule filling to obtain the controlled-release capsules. According to the invention, in-vitro release is uniform, the controlled release effect can be achieved, the average release rate per 2 hours in an in-vitro release rate test is 10-15%, compared with a traditional controlled release preparation, the sustained and controlled release capsules are more uniform in release, the constant-speed release time is nearly 14 hours, the shelf life of the sustained and controlled release capsules is long and can reach 24 months, the impurity increment in the placing process is small, and the impurity increment is only 0.13% after the sustained and controlled release capsules are placed for 24 months. The sustained and controlled release capsules are high in anti-tumor activity, low in toxicity and low in price, and can be used for treating human hepatoma carcinoma cells (HepG-2), human lung cancer cells (A549), humanbreast cancer cells (MCF-7) and human colon cancer cells (CT26 and HT29).
Owner:JINLIN MEDICAL COLLEGE
Chlorine dioxide generator for the efficient generation of chlorine dioxide in dilute solutions
ActiveUS20150065403A1Limited abilityGood flexibilityWaste water treatment from quariesBiocideChlorine dioxideProcess engineering
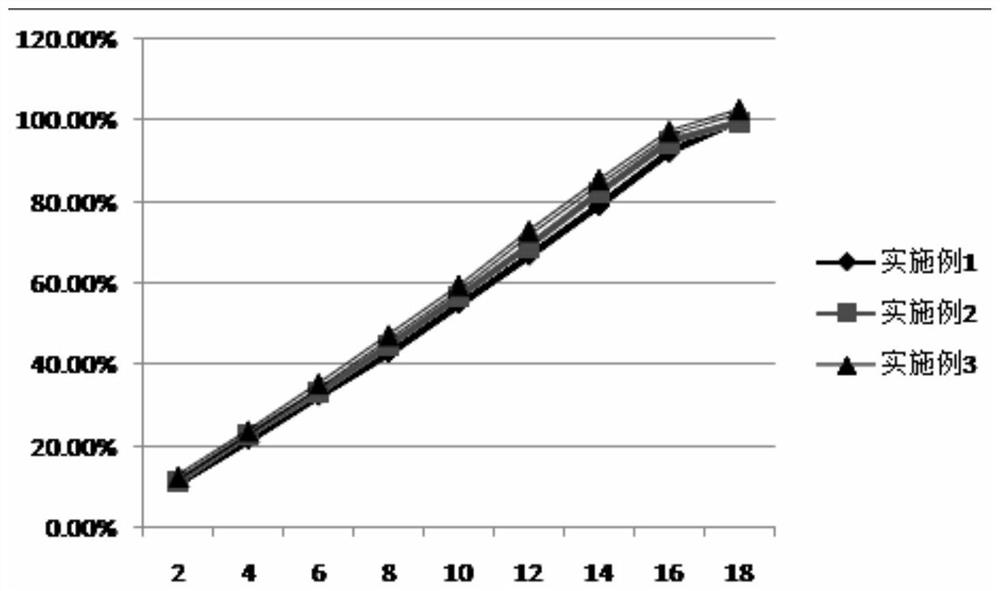
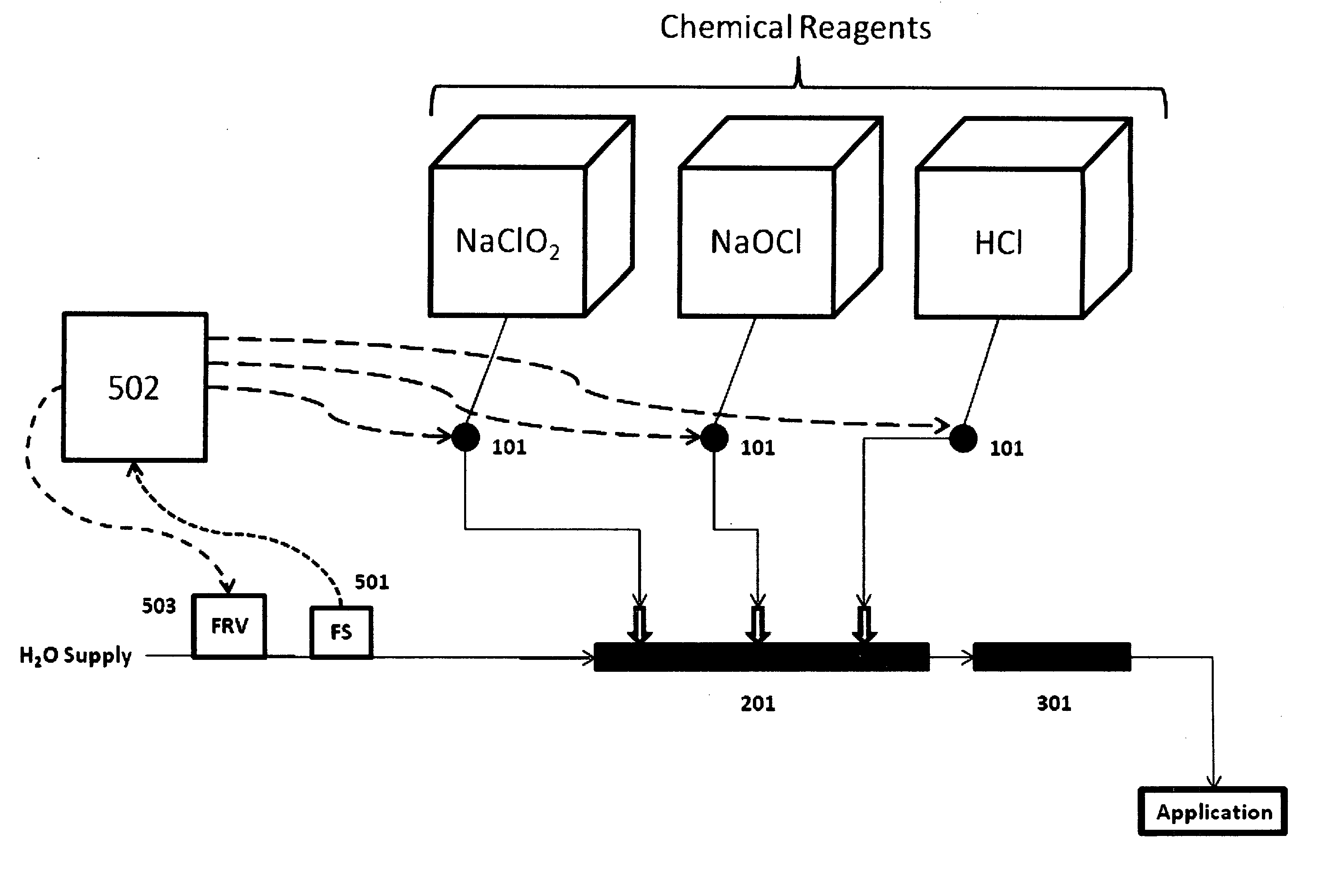
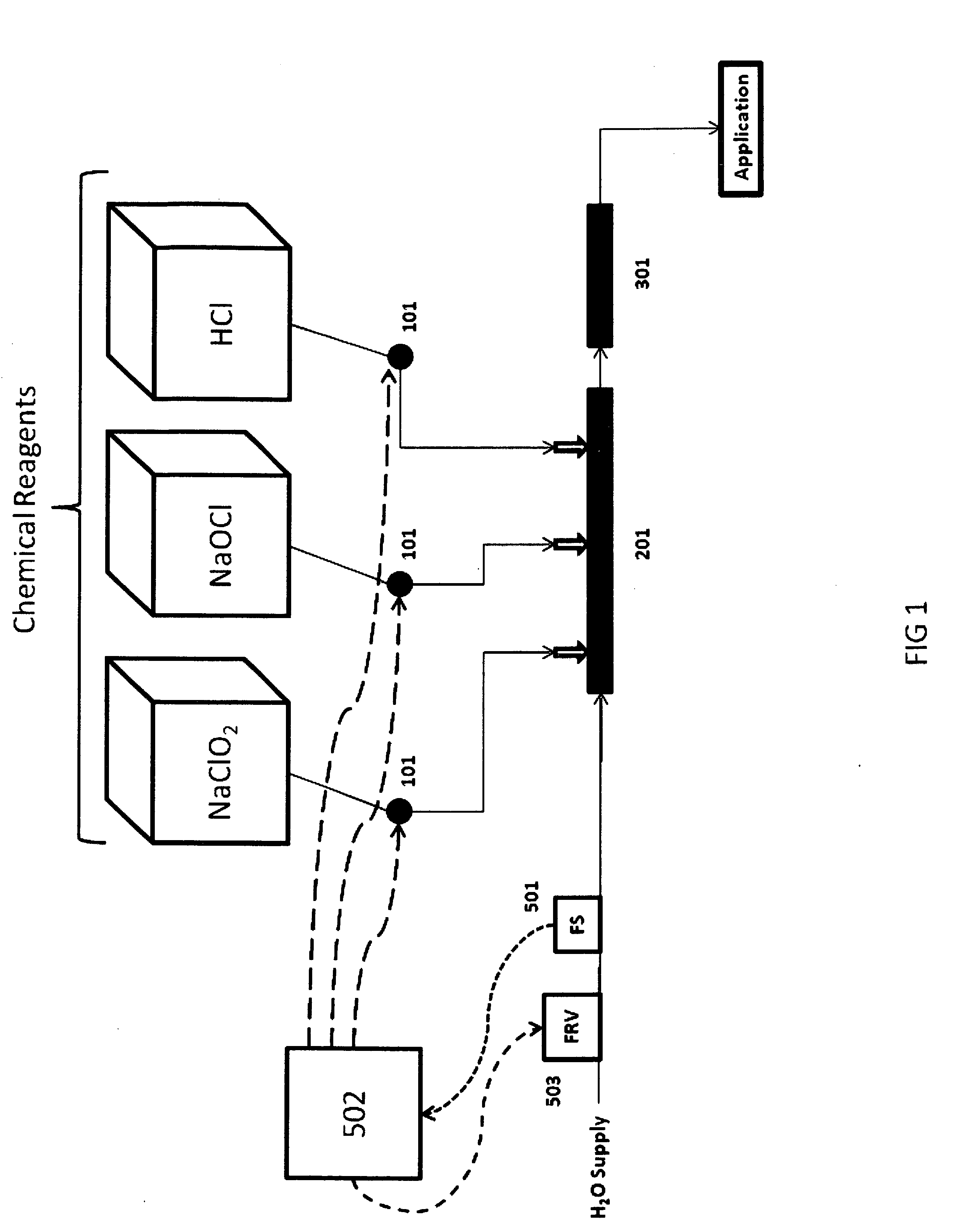
Disclosed is a process for the safe and efficient generation of chlorine dioxide while achieving a variable chlorine dioxide mass flow rate, with a turn-down to turn-up ratio of at least 1 to 200. The process allows for a single chlorine dioxide generating system to safely provide variable mass flow rate of chlorine dioxide to applications that have wide ranging chlorine dioxide demand, like those experienced in oil and gas applications.
Owner:TRUOX
Sub-assembly of external parts for watch
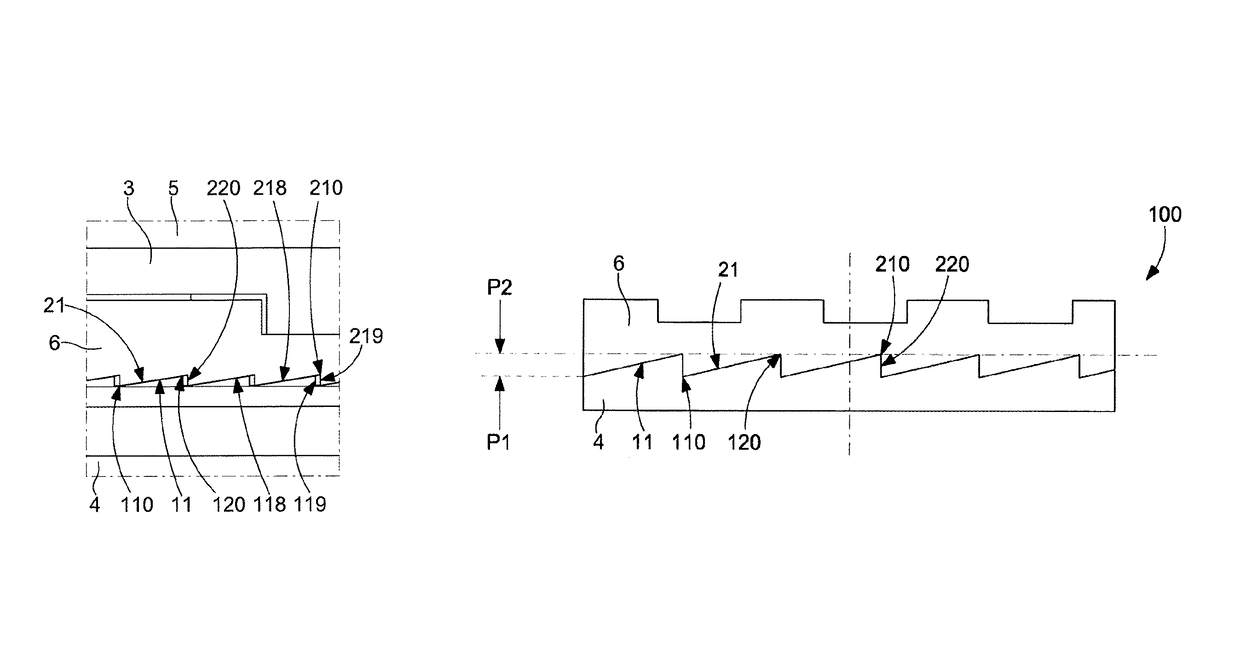
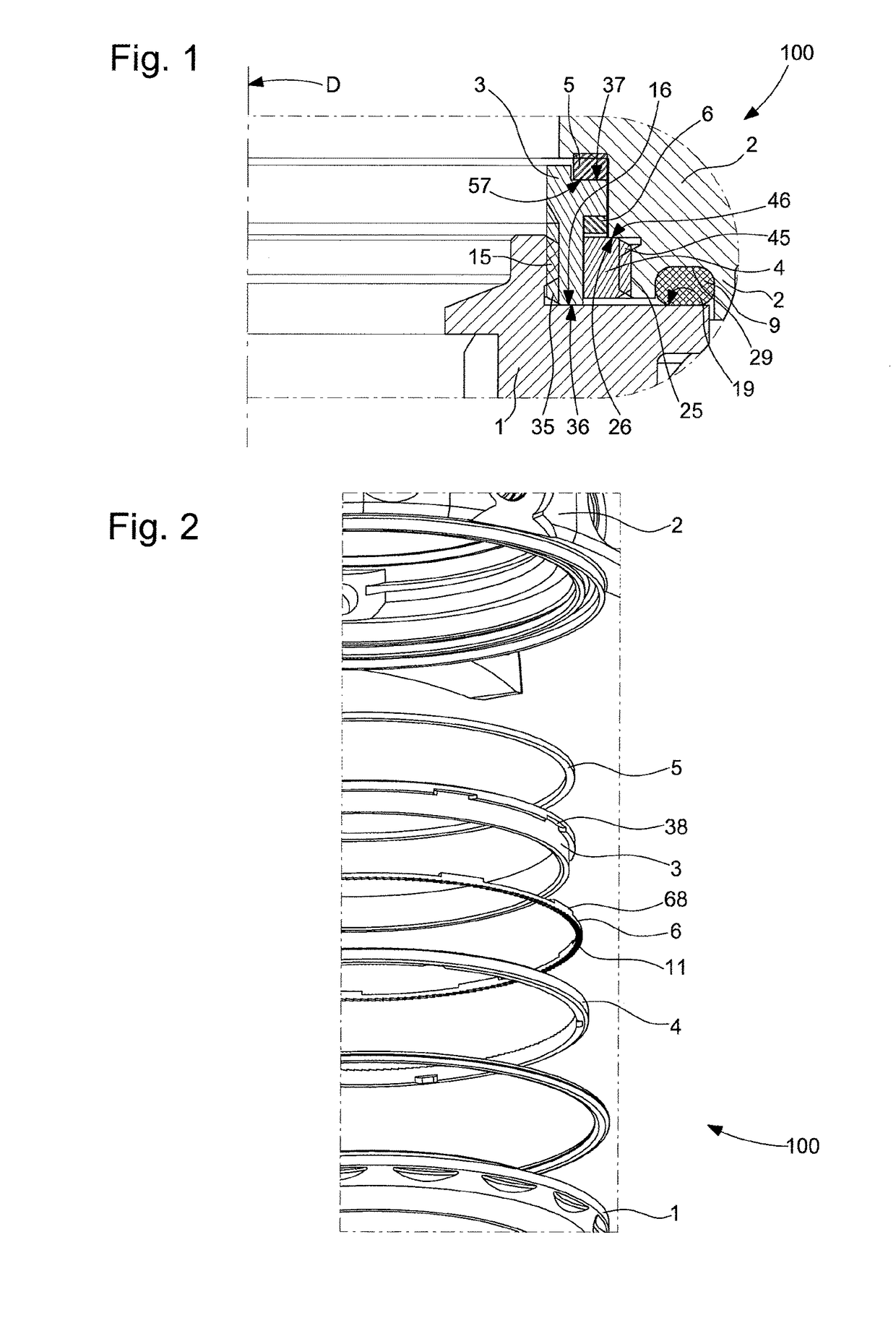
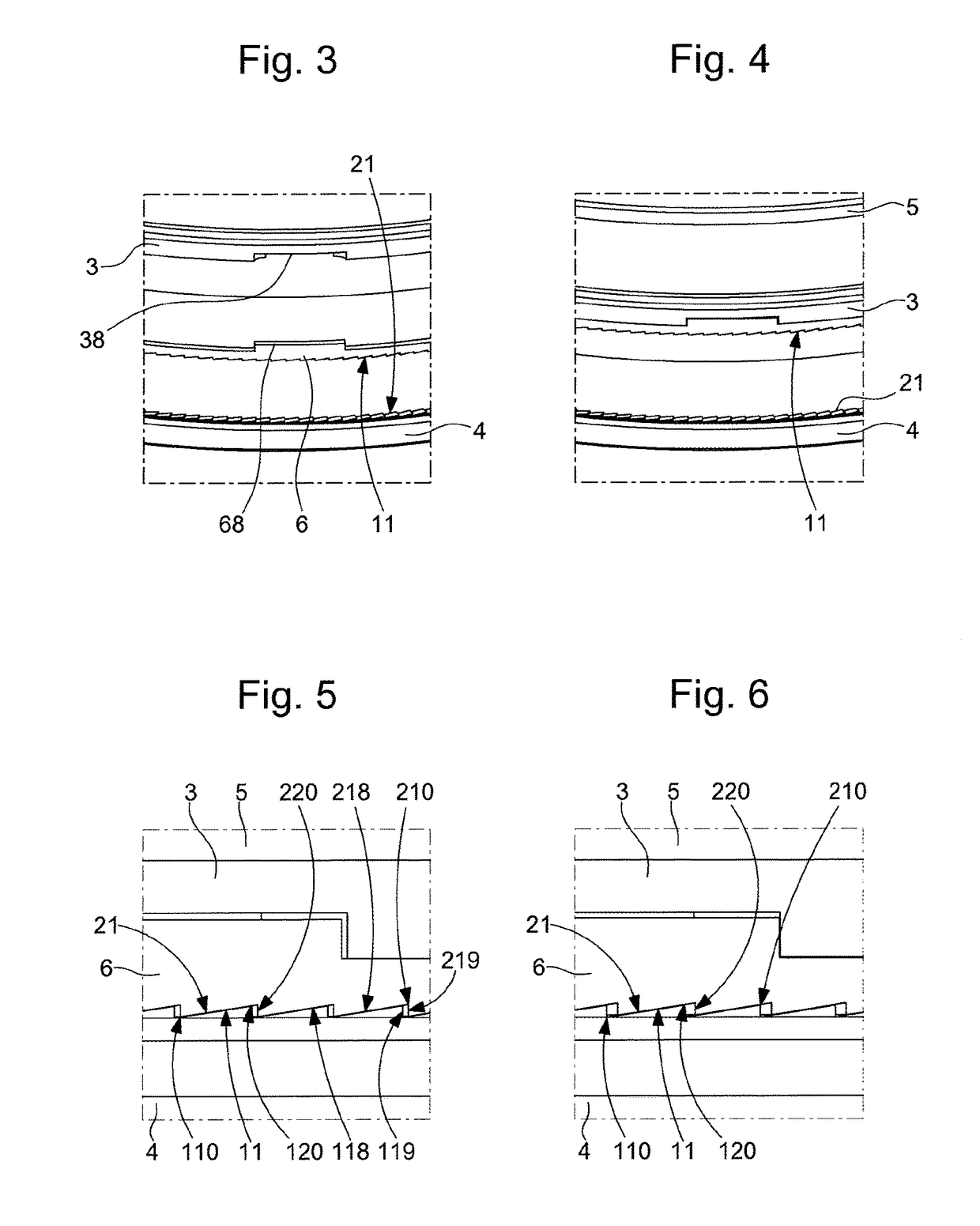
ActiveUS10073418B2Easy to superviseSmall incrementVisual indicationClockwork casesEngineeringWater resistant
Sub-assembly of external parts for a watch, comprising a water-resistant joint between a first and a second component, which are screwed, one on the other, the first component supports a first inclined toothing, which cooperates with a second inclined toothing of the second component, the first component or second component, or a second ring interposed between them comprises a zone which is elastically deformable under the action of a relative rotation between the first and the second component which is caused by an external force, and the deformation range of which allows compression of the elastically deformable zone allowing clearance of the teeth tips during the relative rotation and a release of this zone, allowing return to cooperation of the tips and the hollows of the teeth in a new relative angular position.
Owner:OMEGA SA
Medicine preparation for treating malignant tumors and preparation method thereof
ActiveCN108853030AFast absorptionQuick effectPharmaceutical non-active ingredientsGranular deliveryProduction effectAdhesive
The invention relates to a medicine preparation for treating malignant tumors. The medicine preparation is prepared by the following steps of using ricarpa phosphate as the raw material, and adding acertain amount of filling agent, flavoring agent, adhesive, lubricant, cosolvent, antioxidant and the like; pretreating, pre-mixing, forming particles, drying, arranging particles, totally mixing, packaging and the like. The medicine preparation has the advantages that the ricarpa phosphate particle can be quickly absorbed, the effect is quickly taken, the bioavailability is high, and the carryingand taking are convenient; by adopting the special formula and special technology preparation method, the dissolving property is good, the medicine preparation can be completely dissolved within 10s,the defect of moisture absorbing and caking is overcome in the placing process, the stability is good, and the increment of impurities in the storage process is small, and is only 0.09%; the medicinepreparation is qualified in microorganism check, the shelf life is long and can reach 24 months, and the industrialized production effect is realized; after the medicine preparation is sold on market, the market blank can be filled, and the medicine preparation is suitable for being popularized and applied.
Owner:SHANGHAI ZHAOHUI PHARMA
Child safety seat
A child safety seat includes a base having a shell body, a seat shell assembled with the base, the seat shell being adjustable between a plurality of recline positions relative to the base, and a lock mechanism operable to lock the seat shell with the base at any of the recline positions. The lock mechanism includes two latches assembled with the shell body and movable to engage with and disengage from the seat shell, and two release buttons respectively disposed at a left and a right side of the base and respectively coupled with the two latches, each of the two release buttons being independently operable to drive concurrent unlocking displacements of the two latches.
Owner:WONDERLAND SWITZERLAND AG
Method for producing composition for optical material
ActiveUS9458293B2High refractive indexHigh strengthOptical partsOptical elementsInorganic compoundRefractive index
According to the present invention, a composition for optical material, which is capable of providing a homogeneous optical material, can be prepared through pre-polymerization reaction between (a) an inorganic compound having a sulfur atom and (b) an episulfide compound using a hindered amine as a catalyst, followed by mixing with (c) a polythiol compound and (d) a polyisocyanate compound. Moreover, this composition for optical material can be polymerized and cured to thereby provide an optical material having high refractive index (ne of 1.73 or higher), high strength (an elongation of 13% or more in three-point bend test and good drilling resistance), and high heat resistance (a softening point of 70° C. or higher, as measured by TMA).
Owner:MITSUBISHI GAS CHEM CO INC
Anti-freezing sludge deep dehydration conditioner as well as preparation and application methods thereof
The invention relates to an anti-freezing sludge deep dehydration conditioner as well as preparation and application methods thereof, and belongs to the technical field of sludge treatment. The anti-freezing sludge deep dehydration conditioner is prepared from the following components in parts by mass through reaction: 600 to 800 parts of polydimethyldiallyl ammonium chloride, 100 to 200 parts ofethylene glycol, 1 to 5 parts of a cationic guar gum polymer and 5 to 25 parts of polyferric sulfate. After sludge is conditioned by the anti-freezing sludge deep dehydration conditioner, the dehydration performance is obviously improved, and the water content of a filter cake can be reduced to 50-60% from 95-99.9%. The anti-freezing sludge deep dehydration conditioner has the advantages of low freezing point, convenience in transportation, storage and use, high conditioning speed, high sludge dehydration rate, less increment of sludge generated by the conditioner, simplicity in operation, high sludge volume reduction rate and the like, so that the anti-freezing sludge deep dehydration conditioner can be widely applied to sludge conditioning and enhanced deep dehydration.
Owner:山东益源环保科技有限公司
a cs 5 Sife (oh 2 )w 11 o 39 6 hours 2 o Preparation method and application of sustained and controlled release capsule
ActiveCN111904949BUniform release in vitroImprove anti-tumor activityHeavy metal active ingredientsInorganic non-active ingredientsSustained release pelletsEthyl group
a Cs 5 SiFe(OH 2 )W 11 o 39 ·6H 2 The preparation method of O sustained and controlled release capsule is based on Cs 5 SiFe(OH 2 )W 11 o 39 ·6H 2 O as raw material, lactose, ethyl cellulose, hypromellose, silicon dioxide, montmorillonite, sodium chloride, cellulose acetate, dibutyl phthalate, simethicone, magnesium stearate , sodium bisulfite, and sodium lauryl sulfate are used as auxiliary materials, which are respectively prepared by slow-release pellets, wrapped by an adsorption water diversion layer, wrapped by a controlled-release coating layer, and finally filled with capsules; Uniform, can achieve controlled release, the average release rate in the in vitro release test is 10% to 15% every 2 hours, compared with traditional controlled and sustained release preparations, the release is more uniform, the constant release rate is nearly 14 hours, the shelf life of this product Long, up to 24 months, the impurity increase is small during the storage process, and the impurity increase is only 0.13% after 24 months of storage. The invention has high anti-tumor activity, low toxicity, and low price, and can be used for human liver cancer cells (HepG‑2) , human lung cancer cells (A549), human breast cancer cells (MCF‑7), and human colon cancer cells (CT26, HT29).
Owner:JINLIN MEDICAL COLLEGE
Sub-assembly of external parts for watch
ActiveUS20180011445A1Easy to superviseSmall incrementVisual indicationClockwork casesEngineeringWater resistant
Sub-assembly of external parts for a watch, comprising a water-resistant joint between a first and a second component, which are screwed, one on the other, the first component supports a first inclined toothing, which cooperates with a second inclined toothing of the second component, the first component or second component, or a second ring interposed between them comprises a zone which is elastically deformable under the action of a relative rotation between the first and the second component which is caused by an external force, and the deformation range of which allows compression of the elastically deformable zone allowing clearance of the teeth tips during the relative rotation and a release of this zone, allowing return to cooperation of the tips and the hollows of the teeth in a new relative angular position.
Owner:OMEGA SA
A method of treating sludge
InactiveCN103880265BImprove dehydration rateSmall incrementSludge treatment by oxidationChemical oxygen demandFiltration
The invention provides a sludge processing method which comprises the following steps: a) mixing to-be-treated sludge with an oxidant and an oxidation guide catalyst for reaction; and b) mixing a reaction product obtained by the step a) with a coagulation agent for filtration. The oxidation guide catalyst comprises the following components: 5wt%-45wt% of a metal compound and 55wt%-95wt% of an adsorbent. According to the method, firstly the to-be-treated sludge is mixed with the oxidant and the oxidation guide catalyst for oxidation reaction, and the obtained reaction product is mixed with the coagulation agent for filtration, so that the sludge processing is completed. According to the method, hydrogen peroxide and ozone is used as the oxidant, and the oxidation guide catalyst is added, so that membrane breaking and decomposition of microbial extracellular polymers in sludge can be facilitated, the content of reduction materials in the sludge can be reduced, and chemical oxygen demand of the treated sludge is reduced.
Owner:湖南清和环保技术有限公司
Pipeline smoke heating device
The invention discloses a pipeline smoke heating device. The heating device is fixedly into a flue, and comprises a secondary smoke channel, a gas rotational flow cavity and a center air channel fromoutside to inside. Inclined gas rotational flow blades are arranged between the outer wall of the outlet of the center air channel and the inner wall of the gas rotational flow cavity. A combustion cavity is formed in the front ends of the gas rotational flow blades and the center air channel, and a gas rotational flow cavity is formed in the rear ends of the gas rotational flow blades and the center air channel. The gas pipeline communicates with the gas rotational flow cavity, and is filled with the gas. A burning torch penetrates through outer layer walls and is inserted into the combustioncavity. A plurality layers of inclined smoke rotational blades are arranged between the outer wall of the gas rotational flow cavity and the inner wall of the secondary smoke channel. Oxygen in the smoke is adopted to complete combustion and release heat to increase the temperature of the smoke. The shape and size of flame are controlled by the rotational flow blades, and therefore the length ofthe flame is reasonable and the radius of the flame is less than the diameter of a chimney. Damage caused by superheat of the flue is avoided. The temperature rising process of the smoke is completedby the heat exchanging between cold smoke and hot smoke. The process is direct, the cost is saved, energy is saved, and efficiency is achieved.
Owner:WUHAN UNIV OF TECH +1
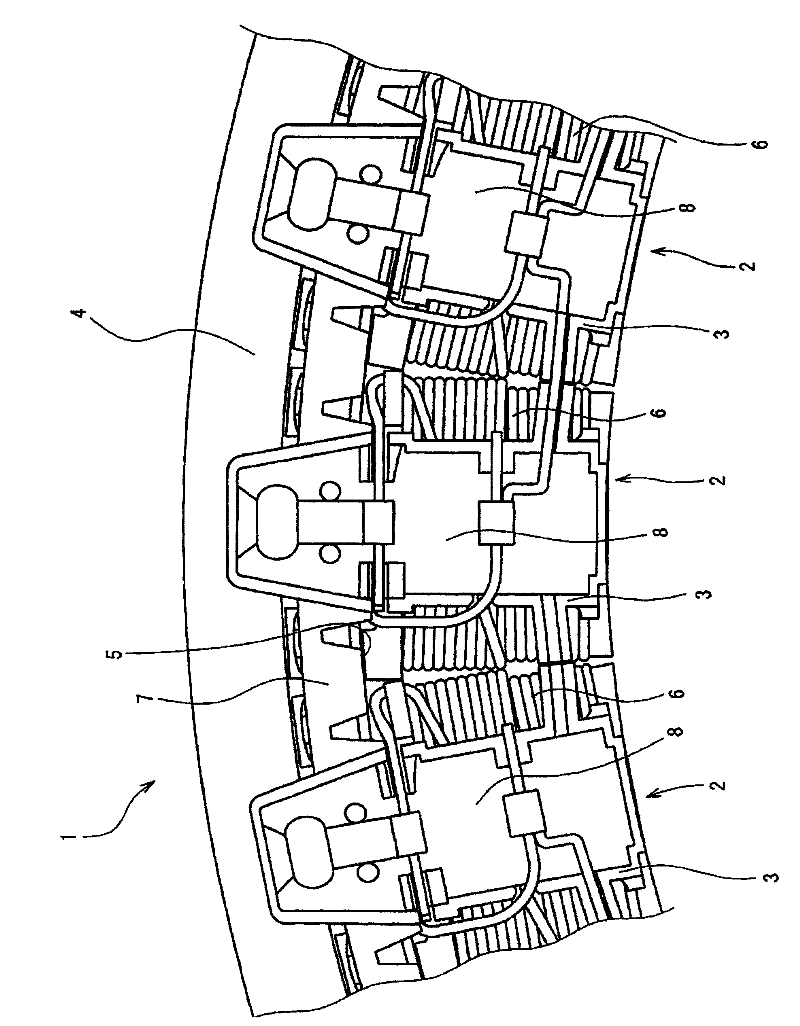
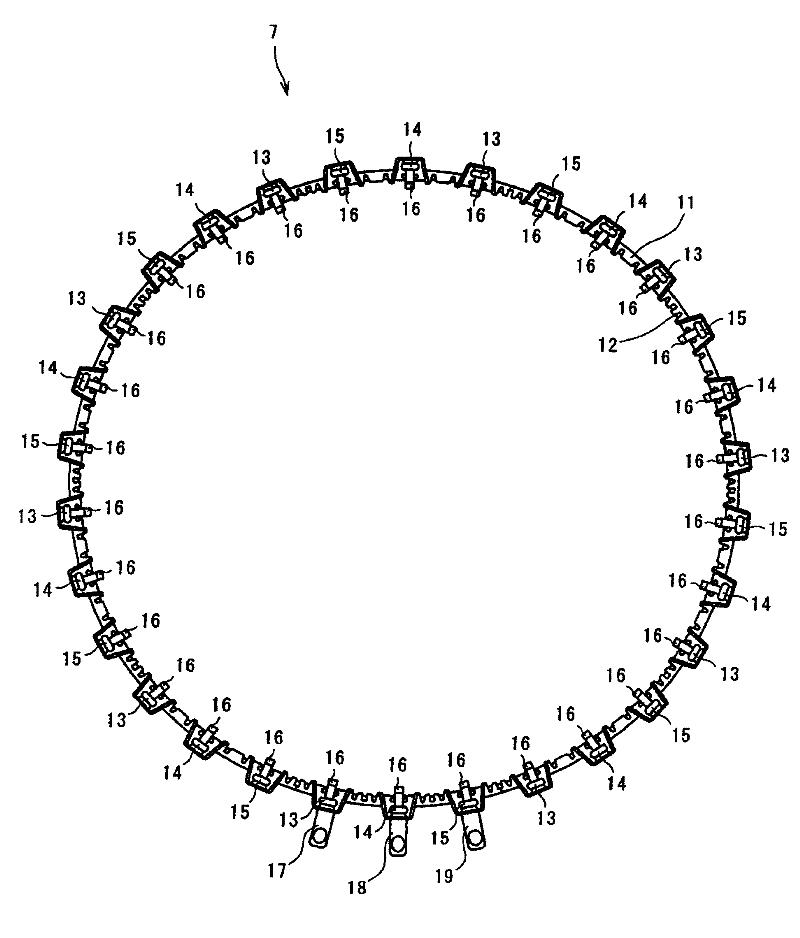
Wiring parts for automotive rotating electrical machines
InactiveCN102290902ASmall incrementExcellent bending workabilityWindings conductor shape/form/constructionElectric machineEngineering
The invention discloses a wiring part of a rotating electrical machine for a vehicle, comprising segmental metals (27, 28, 29) made of conductive wires and arranged in the circumferential direction (26) of the rotating electrical machine, wherein the segmental metals (27 , 28, 29) include: a main body (41), which has a first straight portion (411); a second straight portion (451), which passes through each end of the main body (41) in the radial direction of the rotating electrical machine ( 21) is formed by bending so as to have an obtuse angle (44) between the first straight portion (411) and the second straight portion (451); and, the upright portion (31) at the second straight portion (451) It is formed by bending in the rotation axis direction (25) of the electric rotating machine, and wherein the upstanding portions (31) of the segment metals (27, 28, 29) adjacent to each other are made electrically conductive to each other.
Owner:AISIN SEIKI KK
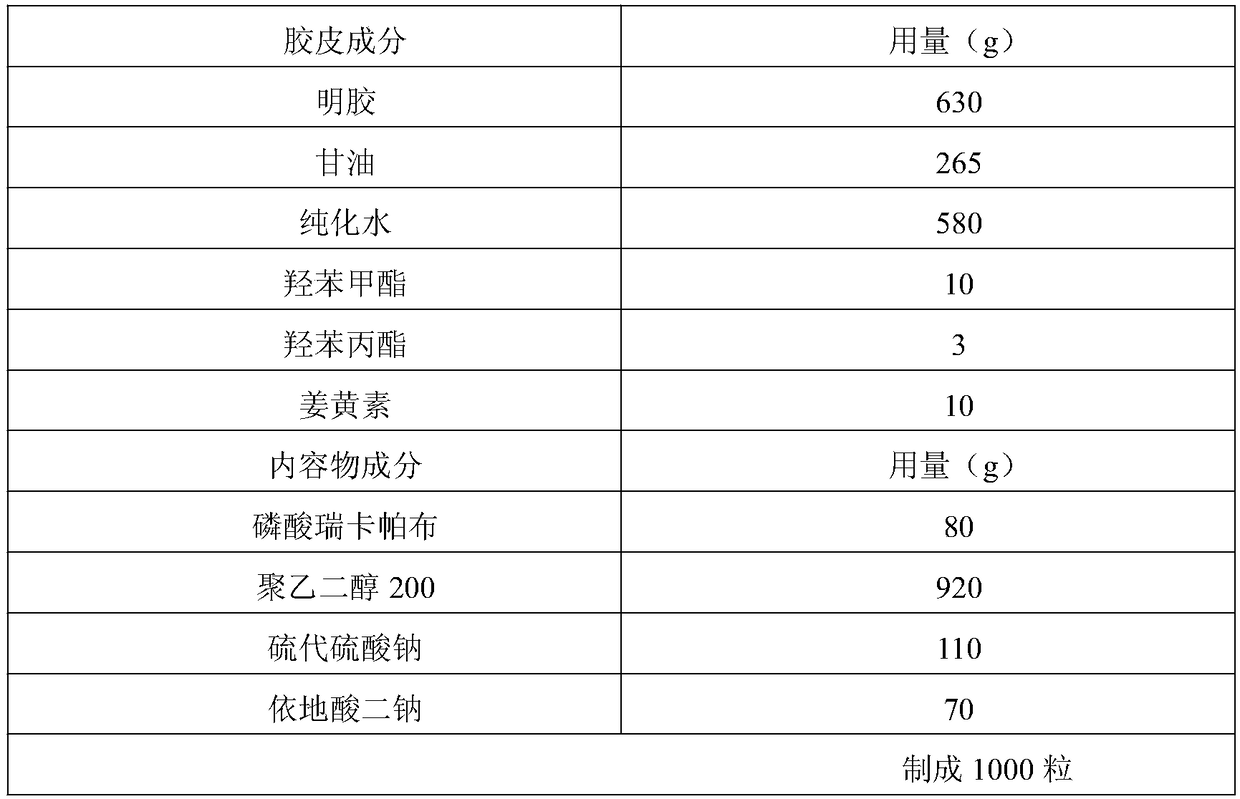
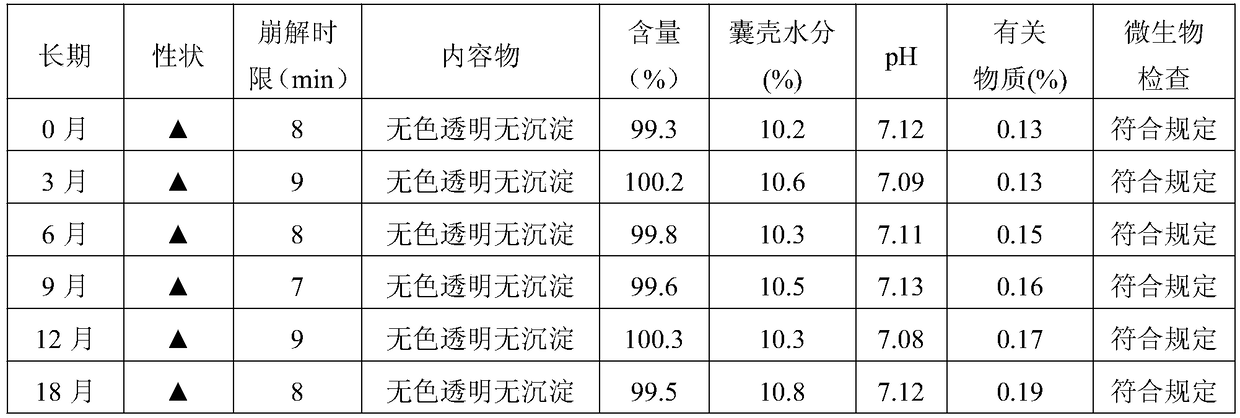
Rucaparib phosphate soft capsule and preparation method thereof
InactiveCN108743557AQuick effectImprove bioavailabilityInorganic non-active ingredientsCapsule deliveryPreservativeMedicine
The invention discloses a rucaparib phosphate soft capsule. The rucaparib phosphate soft capsule comprises a capsule shell and content encapsulated in the capsule shell. The capsule shell comprises gelatin, glycerin, purified water, a preservative and a coloring agent. The content comprises rucaparib phosphate, polyethylene glycol 200 and an antioxidant. Through the prescription and process adjustment, the rucaparib phosphate soft capsule produces effects fast and has high bioavailability. Through the stability inspection test, the rucaparib phosphate soft capsule has good stability. In storage, the capsule shell does not lose moisture so that the capsule shell hardens and ruptures. The pH of the content is decreased and there is no obvious change in pH after 24 months of storage. The change of related substances in the long-term storage process is small. The preparation method is simple and feasible, and is worthy of promotion and application in the market.
Owner:李莉
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com