Medicine preparation for treating malignant tumors and preparation method thereof
A technology of granules and fillers, which is applied in the field of recaprabib phosphate granules and its preparation, can solve the problems of difficult control of microbial limit indicators, poor content uniformity during storage, and easy generation of degraded impurities in products, so as to fill the gap in the market , Easy to carry and take, quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
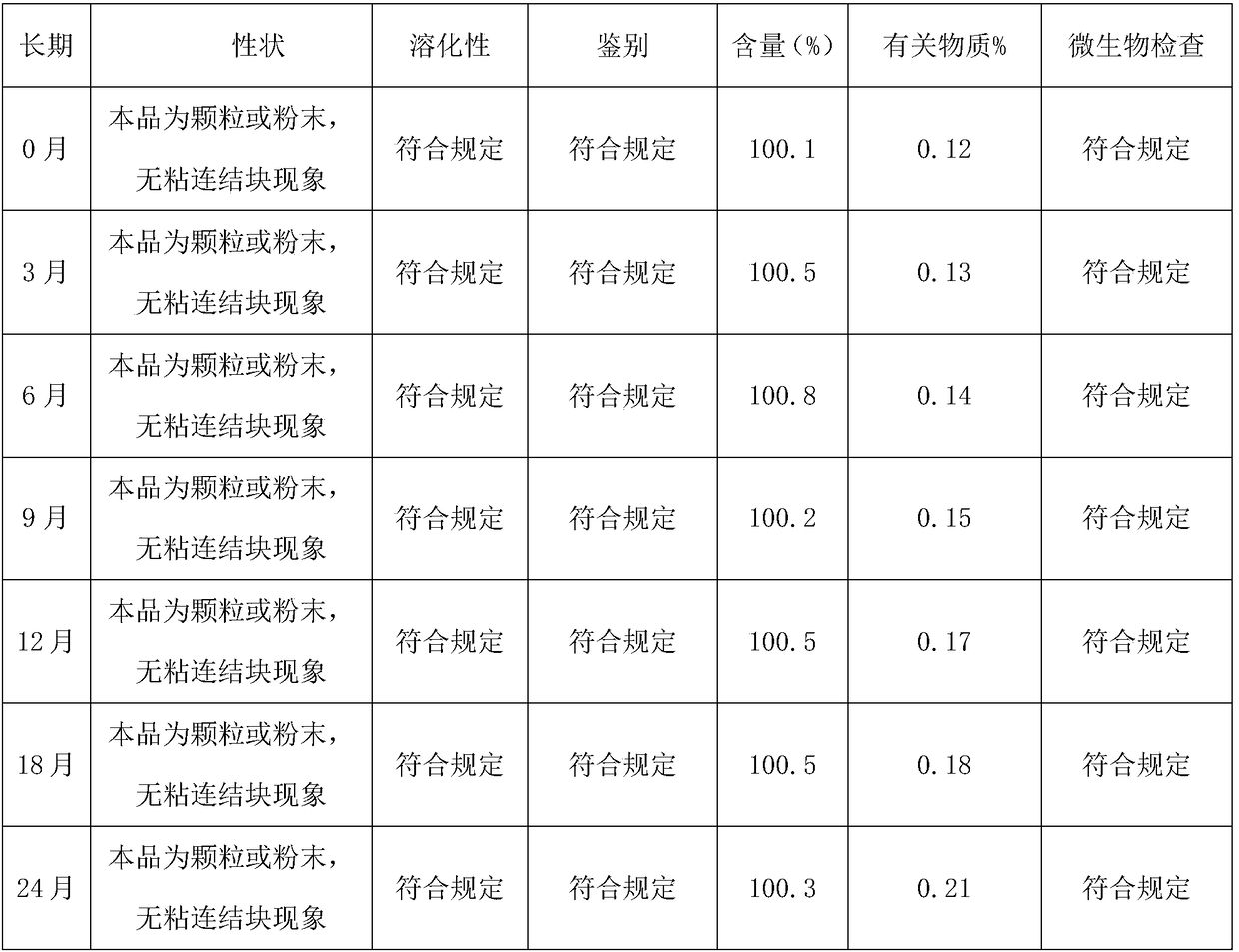
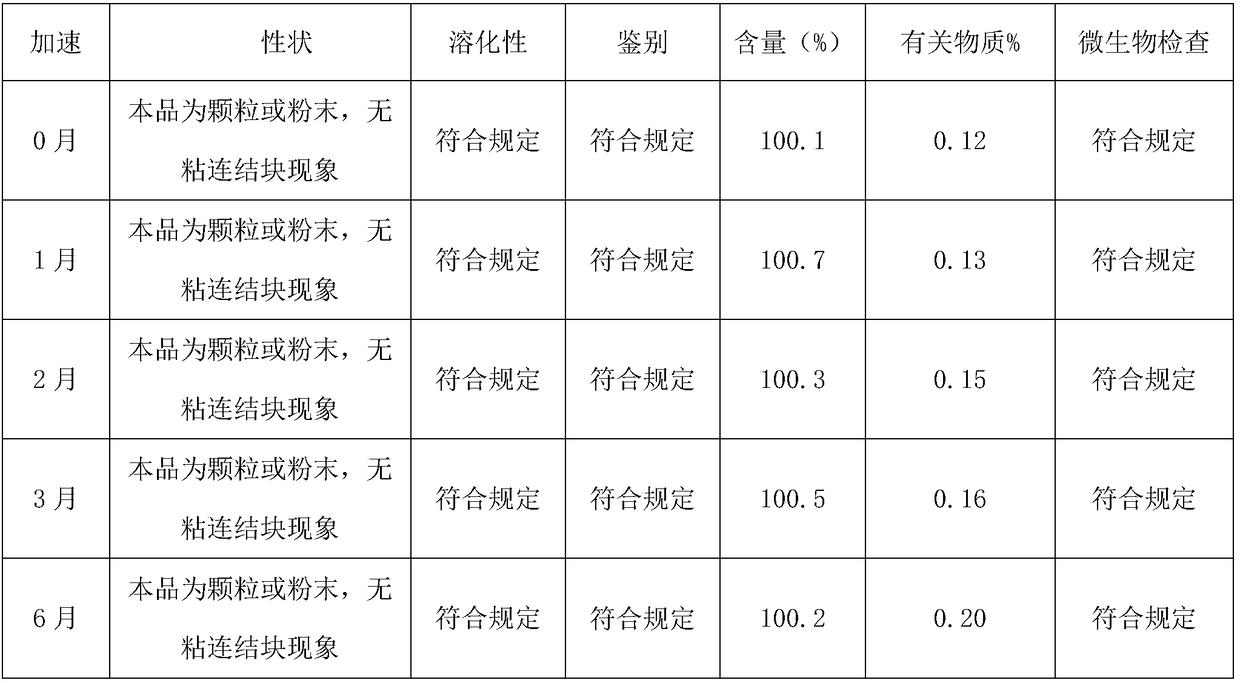
Image
Examples
Embodiment 1
[0024] A granule of recaprabib phosphate:
[0025] Prescription: 1 part of recaprabib phosphate, 1.0 part of lactose, 1.4 parts of microcrystalline cellulose, 0.9 part of sodium carboxymethyl starch, 0.7 part of mannitol, 0.05 part of aspartame, and polyvinylpyrrolidone with a mass fraction of 7% 1.6 parts of ethanol solution, 0.04 parts of magnesium stearate, 0.5 parts of Tween 20, 0.7 parts of Span 80, 0.05 parts of vitamin C, and 0.14 parts of methionine;
[0026] Preparation:
[0027] 1. Pretreatment of raw and auxiliary materials: Take the prescribed amount of recaprabib phosphate, lactose, microcrystalline cellulose, sodium carboxymethyl starch, mannitol, aspartame, vitamin C, and methionine; In the pulverizer, pulverize respectively through a 100-mesh sieve, and set aside;
[0028] 2. Premixing: Put the pulverized recaparib phosphate, lactose, microcrystalline cellulose, sodium carboxymethyl starch, mannitol, aspartame, vitamin C, and methionine in the three-dimension...
Embodiment 2
[0054] A granule of recaprabib phosphate:
[0055] Prescription: 1 part of recaprabib phosphate, 0.9 parts of lactose, 1.3 parts of microcrystalline cellulose, 0.8 parts of carboxymethyl starch sodium, 0.6 parts of mannitol, 0.04 parts of aspartame, and polyvinylpyrrolidone with a mass fraction of 7% 1.5 parts of ethanol solution, 0.03 parts of magnesium stearate, 0.4 parts of Tween 20, 0.6 parts of Span 80, 0.04 parts of vitamin C, and 0.13 parts of methionine;
[0056] Preparation:
[0057] 1. Pretreatment of raw and auxiliary materials: Take the prescribed amount of recaprabib phosphate, lactose, microcrystalline cellulose, sodium carboxymethyl starch, mannitol, aspartame, vitamin C, and methionine; In the pulverizer, pulverize respectively through a 100-mesh sieve, and set aside;
[0058] 2. Premixing: Put the pulverized recaparib phosphate, lactose, microcrystalline cellulose, sodium carboxymethyl starch, mannitol, aspartame, vitamin C, and methionine in the three-dimen...
Embodiment 3
[0066] A granule of recaprabib phosphate:
[0067] Prescription: 1 part of recaprabib phosphate, 1.1 parts of lactose, 1.5 parts of microcrystalline cellulose, 1.0 parts of sodium carboxymethyl starch, 0.8 parts of mannitol, 0.06 parts of aspartame, and polyvinylpyrrolidone with a mass fraction of 8% 1.7 parts of ethanol solution, 0.05 parts of magnesium stearate, 0.6 parts of Tween 20, 0.8 parts of Span 80, 0.06 parts of vitamin C, and 0.15 parts of methionine;
[0068] Preparation:
[0069] 1. Pretreatment of raw and auxiliary materials: Take the prescribed amount of recaprabib phosphate, lactose, microcrystalline cellulose, sodium carboxymethyl starch, mannitol, aspartame, vitamin C, and methionine; In the pulverizer, pulverize respectively through a 100-mesh sieve, and set aside;
[0070] 2. Premixing: Put the pulverized recaparib phosphate, lactose, microcrystalline cellulose, sodium carboxymethyl starch, mannitol, aspartame, vitamin C, and methionine in the three-dimen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com