Preparation method and application of Cs5SiFe (OH2) W11O39. 6H2O sustained and controlled release capsules
A technology of capsules and sustained-release pellets, which is applied in the field of medicine, can solve the problems that anti-tumor drugs have not been reported, and there is no Keggin-type silicotungstate anti-tumor preparation, etc., and achieve small fluctuations in blood drug concentration, low price, and safety sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
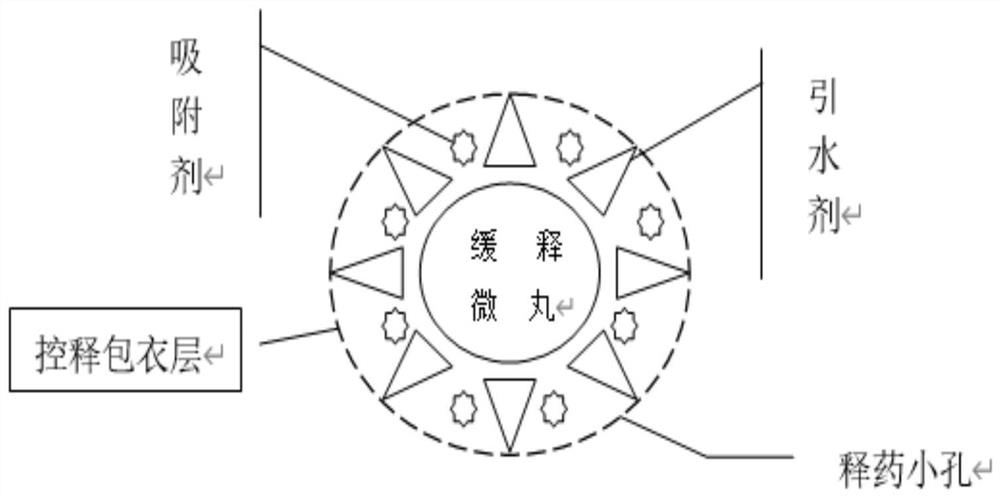
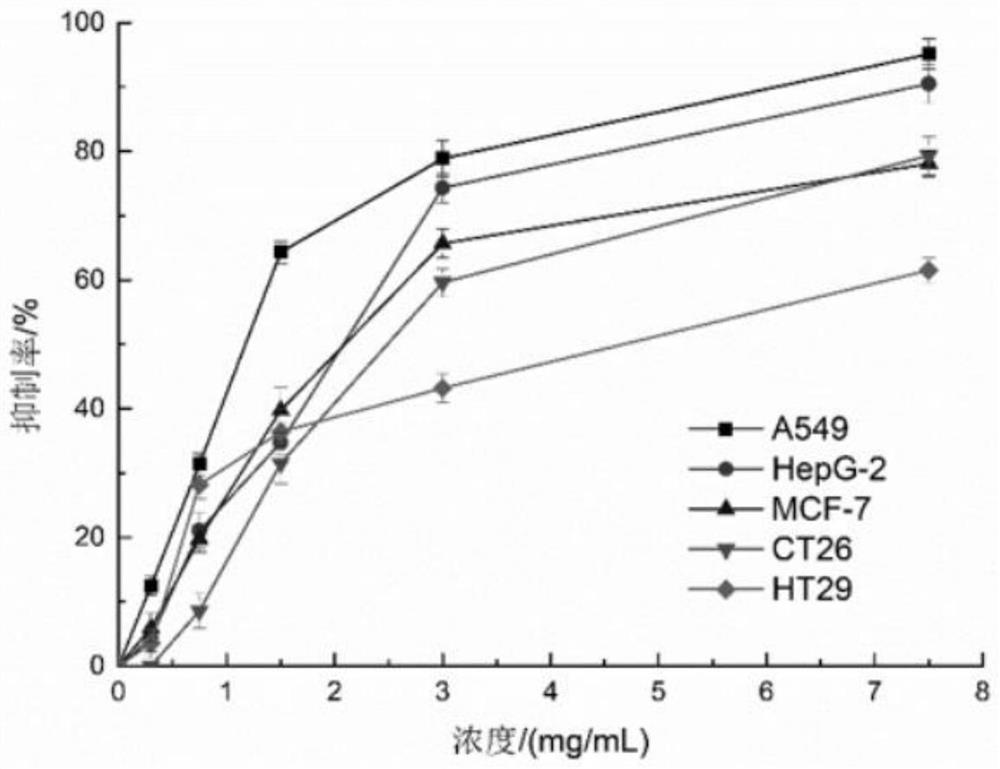
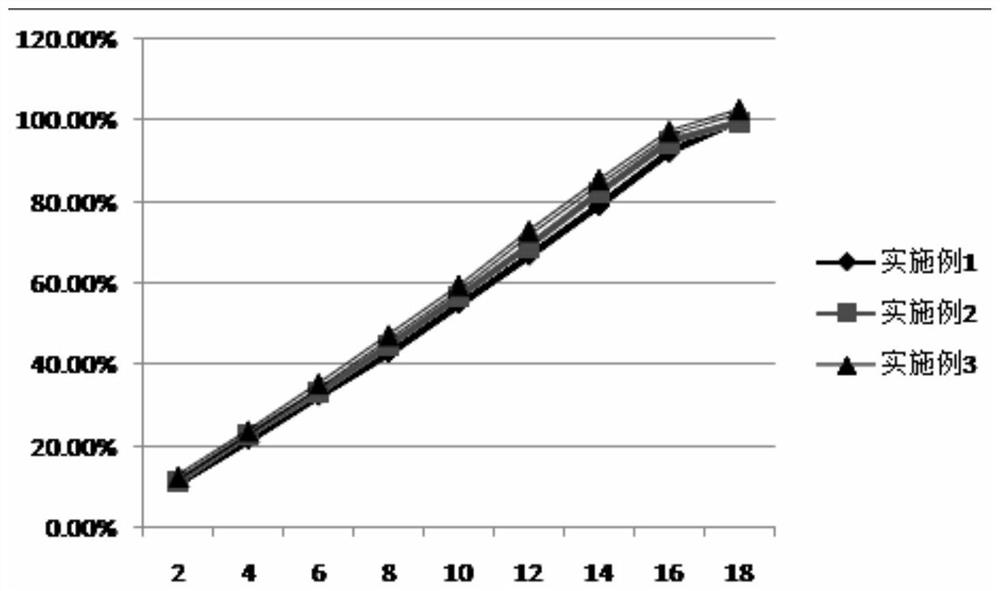
Image
Examples
Embodiment 1
[0022] a Cs 5 SiFe(OH 2 )W 11 o 39 ·6H 2 The preparation method of O sustained and controlled-release capsules is prepared in the following steps:
[0023] 1.K 8 SiW 11 o 39 13H 2 Preparation of O:
[0024] (1) Weigh 1.1 kg (5 mol) of sodium metasilicate and place it in a beaker, add 10 L of distilled water, stir and dissolve to obtain solution A;
[0025] (2) Add 1.82kg sodium tungstate (55mol) into 30L boiled distilled water, stir and dissolve to obtain solution B, then add 16.5L hydrochloric acid (4mol / L) to solution B within 30min, stir to dissolve, and then add the solution A, and quickly add 5L hydrochloric acid (4mol / L) to make the solution pH value 5.8, continue to stir and keep the solution boiling for 1 hour, then cool to room temperature, filter, then add 15kg potassium chloride, then continue to stir until white precipitate is generated , filter, collect the white precipitate, then wash the white precipitate with 5L saturated potassium chloride solution, ...
Embodiment 2
[0044] a Cs 5 SiFe(OH 2 )W 11 o 39 ·6H 2 The preparation method of O sustained and controlled-release capsules is prepared in the following steps:
[0045] 1. The preparation of slow-release pellets: get the Cs that 30g embodiment 1 makes 5 SiFe(OH 2 )W 11 o 39 ·6H 2 O, 80g lactose, 30g ethyl cellulose, 20g hypromellose, and 20g silicon dioxide were respectively pulverized through a 100-mesh sieve, then placed in a three-dimensional motion mixer, mixed for 18 minutes to obtain a mixed powder, and set aside; take another volume 2000g of ethanol solution with a fraction of 65%, slowly add the above mixed powder under stirring condition, continue to stir for 60 minutes to disperse completely, and obtain the mixed powder dispersion liquid for later use; put the above mixed powder dispersion liquid in spray drying Spray granulation is carried out in the machine, the air inlet temperature is set at 100-110°C, the rotation speed of the peristaltic pump is 2.0-3.5r / min, and t...
Embodiment 3
[0050] a Cs 5 SiFe(OH 2 )W 11 o 39 ·6H 2 The preparation method of O sustained and controlled-release capsules is prepared in the following steps:
[0051] 1. The preparation of slow-release pellets: get the Cs that 60g embodiment 1 makes 5 SiFe(OH 2 )W 11 o 39 ·6H 2 O, 130g lactose, 70g ethylcellulose, 50g hypromellose, and 40g silicon dioxide were pulverized through a 100-mesh sieve respectively, then placed in a three-dimensional motion mixer, mixed for 22 minutes to obtain a mixed powder, and set aside; take another volume 3000g of ethanol solution with a fraction of 65%, slowly add the above mixed powder under stirring condition, continue to stir for 80 minutes to disperse completely, and obtain the mixed powder dispersion liquid for later use; put the above mixed powder dispersion liquid in spray drying Spray granulation is carried out in the machine, the air inlet temperature is set at 100-110°C, the rotation speed of the peristaltic pump is 2.0-3.5r / min, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com