Preparation method and application of keggin type silicotungstate sustained-release capsules
A technology of silicotungstic acid salt and capsule, which is applied in the field of medicine and achieves the effects of small impurity increment, reduced toxic and side effects, and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of anti-cancer Keggin type silicotungstate sustained-release capsules, prepared according to the following steps:
[0028] 1.K 8 SiW 11 o 39 13H 2 Preparation of O:
[0029] (1) Weigh 1.1 kg (5 mol) of sodium metasilicate and place it in a beaker, add 10 L of distilled water, stir and dissolve to obtain solution A;
[0030] (2) Add 1.82kg sodium tungstate (55mol) into 30L boiled distilled water, stir and dissolve to obtain solution B, then add 16.5L hydrochloric acid (4mol / L) to solution B within 30min, stir to dissolve, and then add the solution A, and quickly add 5L hydrochloric acid (4mol / L) to make the solution pH value 5.0~6.0, continue to stir and keep the solution boiling for 1 hour, then cool to room temperature, filter, then add 15kg potassium chloride, and then continue to stir until White precipitate, filter, collect the white precipitate, then wash the white precipitate with 5L saturated potassium chloride solution, wash twice, wash...
Embodiment 2
[0049] A preparation method of anti-cancer Keggin type silicotungstate sustained-release capsules, prepared according to the following steps:
[0050] 1. Rb 5 SiFe(OH 2 )W 11 o 39 ·7H 2 O(RbSiW 11 Fe) Preparation:
[0051] Prepare K by the preparation method of Example 1 5 SiFe(OH 2 )W 11 o 39 14H 2 O, then K 5 SiFe(OH 2 )W 11 o 39 14H 2 Dissolve O in distilled water. After the dissolution is complete, cool to 2°C, and then pass this solution through H at a temperature of 2±0.5°C. + Type ion exchange resin was exchanged twice. Weigh an appropriate amount of Rb 2 CO 3 , dissolved in ice water, then slowly added to the exchanged acid solution, stirred for 30 minutes, then added an equal volume of 1:3 (v / v) ice methanol-ethanol mixed solution to produce a yellow precipitate, suction filtered, and the product Recrystallize in water at 80°C to achieve the purpose of purifying the product. Collect the precipitate and dry at room temperature to obtain the target p...
Embodiment 3
[0059] A preparation method of anti-cancer Keggin type silicotungstate sustained-release capsules, prepared according to the following steps:
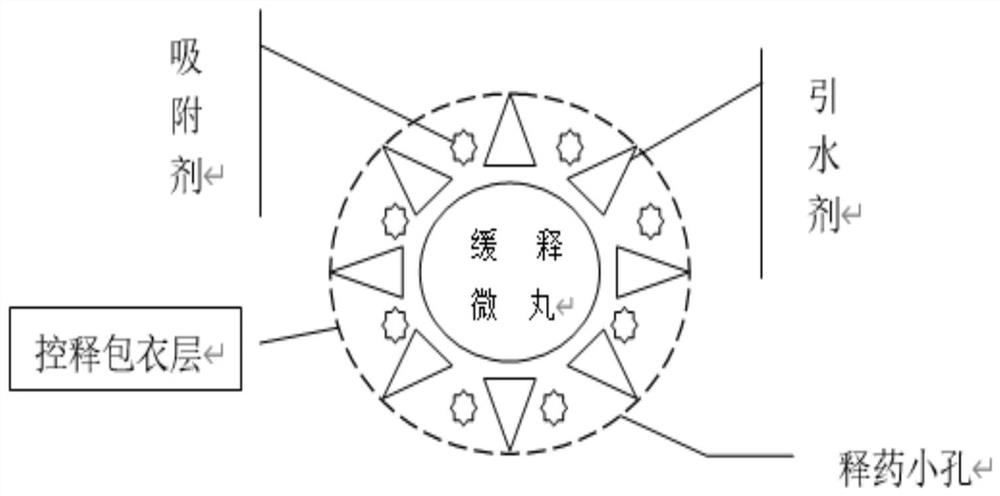
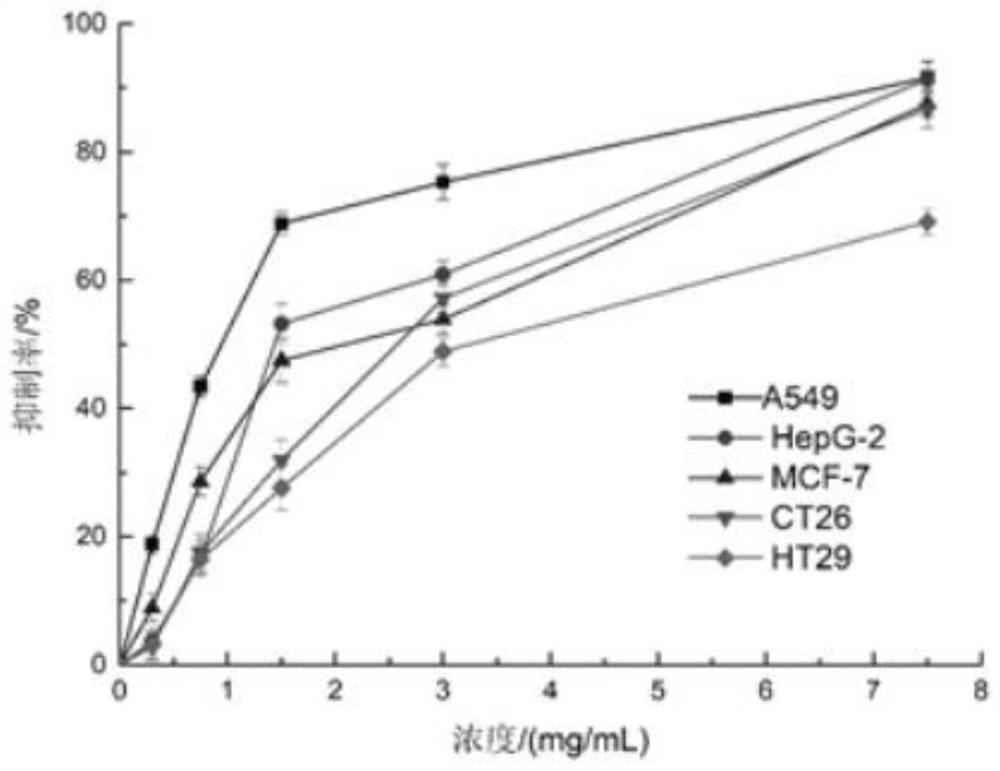
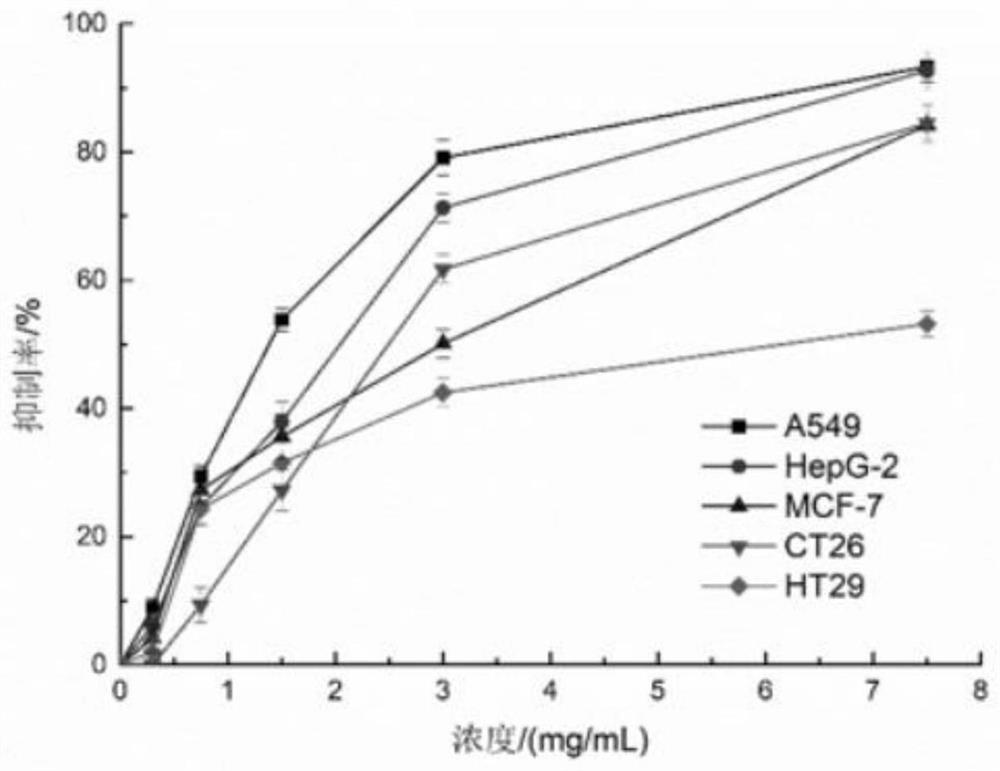
[0060] 1. The preparation of slow-release pellets: get the Rb that embodiment 2 makes 5 SiFe(OH 2 )W 11 o 39 ·7H 2 O80g, lactose 100g, ethyl cellulose 20g, hypromellose 30g, silicon dioxide 10g, then take the above materials and pulverize them through a 100-mesh sieve respectively, mix them and put them in a three-dimensional motion mixer, and mix them for 10 minutes to get a mixed powder , for subsequent use; take another 2000 g of ethanol solution with a volume fraction of 60%, slowly add the above-mentioned mixed powder under stirring conditions, continue to stir for 60 minutes to completely disperse, and obtain a mixed powder dispersion, for subsequent use; the above-mentioned mixed powder The dispersion liquid is placed in a spray dryer for spray granulation, set the inlet air temperature at 100°C, the peristaltic pump speed a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com