Sublingual tablet for anaesthesia and preparation method thereof
A sublingual tablet and prescription technology, applied in the field of anesthetized sublingual tablet and its preparation, can solve the problems of large increase in impurities, poor product stability, poor particle fluidity, etc. Over effect, simple and feasible preparation process, good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A sublingual tablet for anesthesia, prepared according to the following steps:
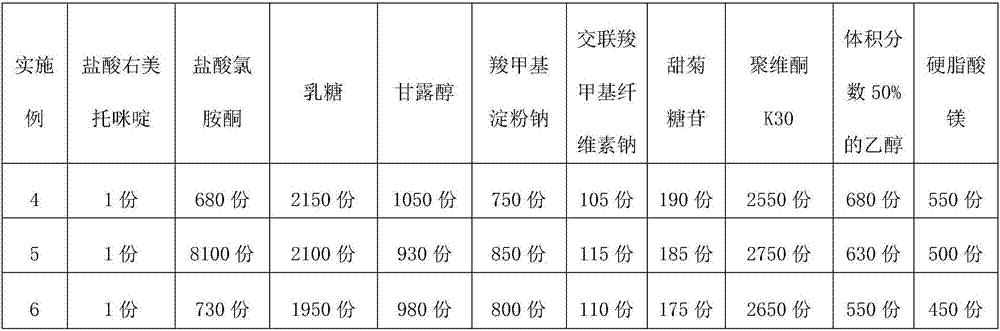
[0027] Element Dosage (parts by weight) Dexmedetomidine Hydrochloride 1 copy Ketamine hydrochloride 1100 copies lactose 1700 copies Mannitol 2950 copies Sodium carboxymethyl starch 600 copies Croscarmellose Sodium 1700 copies steviol glycoside 400 copies Povidone K30 200 copies 50% ethanol by volume 1250 copies Magnesium stearate 400 copies
[0028] Preparation process:
[0029] 1. Pre-treatment: Take the prescribed amount of dexmedetomidine hydrochloride, ketamine hydrochloride, lactose, mannitol, sodium carboxymethyl starch, cross-linked sodium carboxymethyl cellulose, and steviol glycoside, and place them in a universal grinder, Pulverize through a 100-mesh sieve, and set aside; get another prescribed amount of povidone K30 and dissolve it in an ethanol solution with a volume fraction of 50% of the presc...
Embodiment 2
[0063] A sublingual tablet for anesthesia, prepared according to the following steps:
[0064] Element Dosage (parts by weight) Dexmedetomidine Hydrochloride 1 copy Ketamine hydrochloride 900 copies lactose 1600 copies Mannitol 2800 copies Sodium carboxymethyl starch 500 copies Croscarmellose Sodium 1600 copies steviol glycoside 300 copies Povidone K30 150 copies 50% ethanol by volume 1200 copies Magnesium stearate 300 copies
[0065] Preparation process: It is prepared according to the preparation process of Example 1, and the Hugher angle of the particles is measured to be 35°.
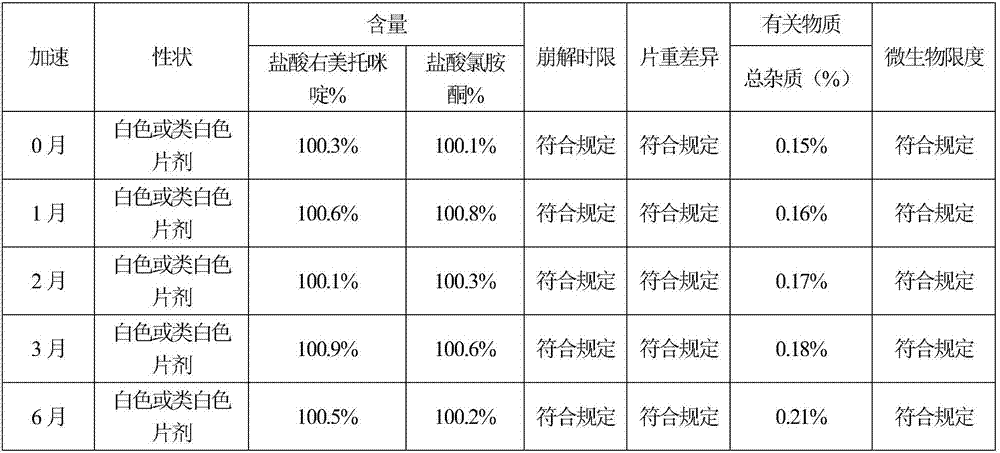
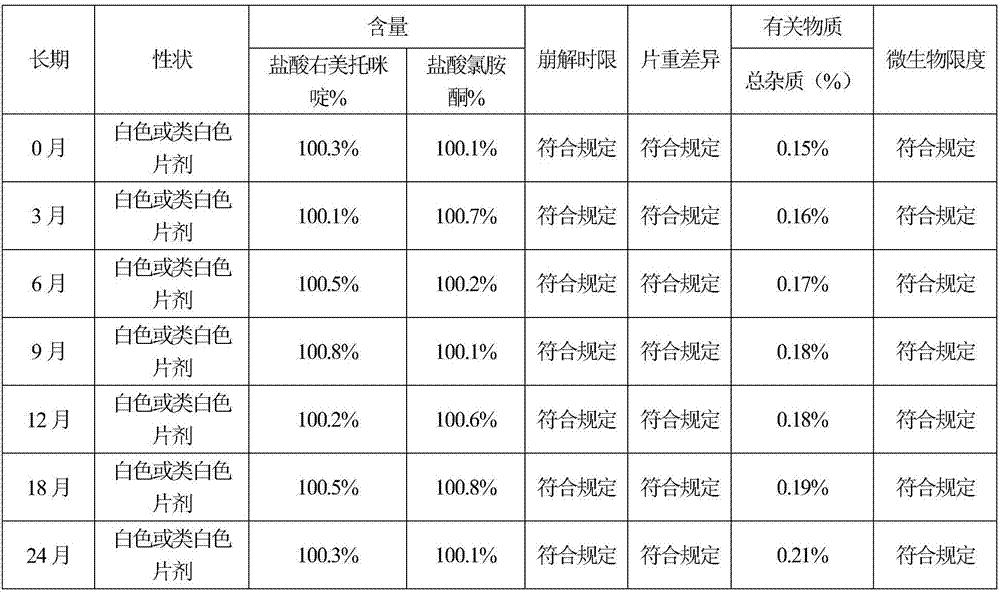
[0066] According to the test method of Example 1, the sample stability test results of Example 2 show that the sample quality is stable in 6 months after acceleration, and the quality is stable for 24 months in a long-term, so the validity period of this product is at least 24 months; the disintegration time limit te...
Embodiment 3
[0068] A sublingual tablet for anesthesia, prepared according to the following steps:
[0069] Element Dosage (parts by weight) Dexmedetomidine Hydrochloride 1 copy Ketamine hydrochloride 1300 copies lactose 1800 copies Mannitol 3100 copies Sodium carboxymethyl starch 700 copies Croscarmellose Sodium 1800 copies steviol glycoside 500 copies Povidone K30 250 copies 50% ethanol by volume 1300 copies Magnesium stearate 500 copies
[0070] Preparation process: It is prepared according to the preparation process of Example 1, and the Hugher angle of the particles is measured to be 33°.
[0071] According to the test method of Example 1, the sample stability test results of Example 3 show that the sample quality is stable in six months after acceleration, and the quality is stable for 24 months in a long-term, so the validity period of this product is at least 24 months; the disintegration time limit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com