Method for detecting specific impurities of mesalazine
A detection method, the technology of mesalazine, which is applied in the field of medicine, can solve the problems of small amount of specific impurities, high operation requirements, and high instrument requirements, and achieve the effects of strong specificity, good precision, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
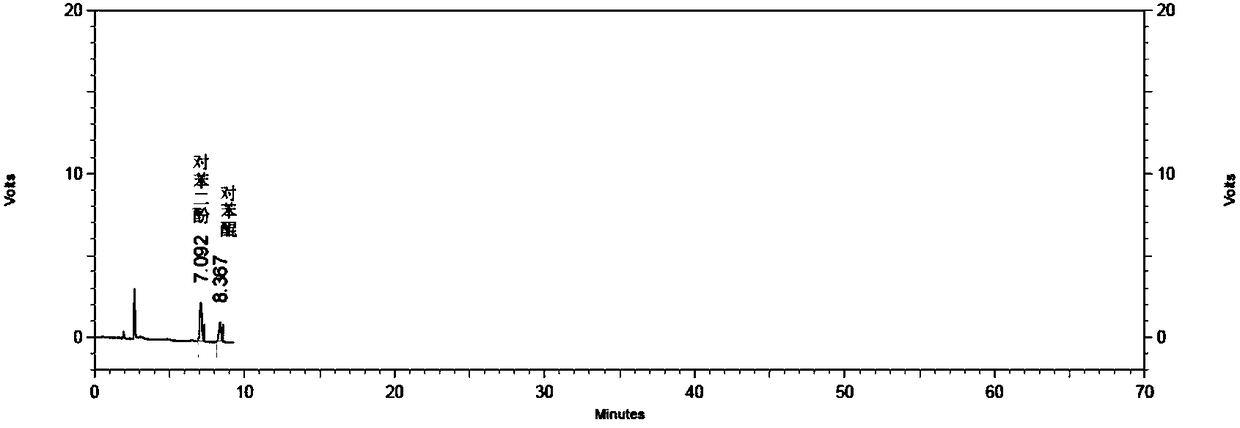
[0045] Using a phenyl-bonded silica gel column, the mobile phase is the aqueous phase (1.36g potassium dihydrogen phosphate and 1.73g sodium octane sulfonate, dissolved in 890ml water, add phosphoric acid to adjust the pH to 2.00)-methanol-acetonitrile-tetrahydrofuran (890 :58:34:18), the flow rate is 1.2ml / min, the detection wavelength is 220nm, and the column temperature is 30°C.
[0046] Weigh various impurity reference substances, add mobile phase to dissolve and dilute to prepare about 100μg / ml each impurity stock solution.
[0047] Hydroquinone and p-benzoquinone reference substance solution: Measure each appropriate amount of hydroquinone and p-benzoquinone stock solution, and dilute with mobile phase to prepare a reference substance containing both hydroquinone and p-benzoquinone about 1μg / ml Solution.
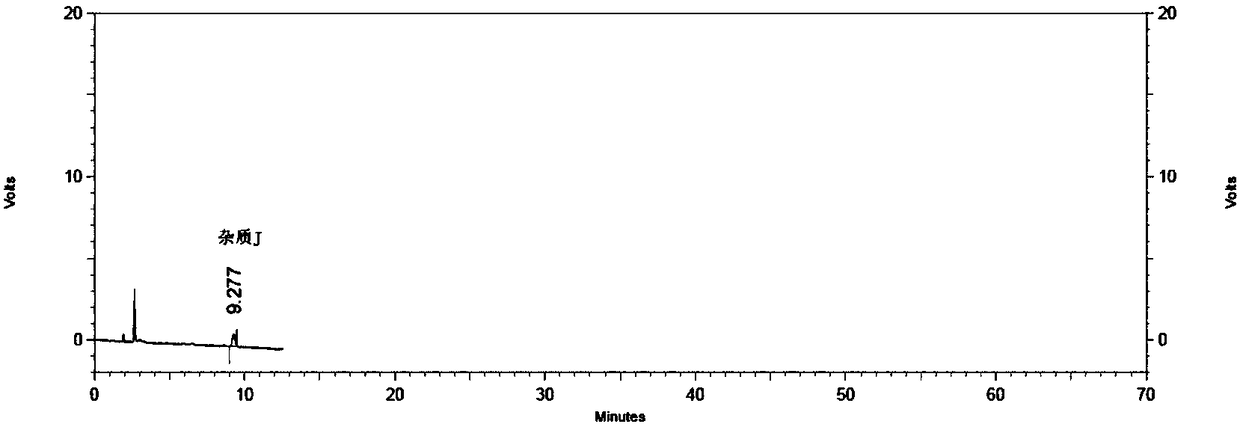
[0048] Impurity J reference solution: Measure an appropriate amount of impurity J stock solution and dilute with mobile phase to prepare a reference solution of about 1μg / ml...
Embodiment 2
[0056] Using a phenyl-bonded silica gel column, the mobile phase is the aqueous phase (2.72g potassium dihydrogen phosphate and 1.73g sodium octane sulfonate, dissolved in 872ml water, add phosphoric acid to adjust the pH to 2.15)-methanol-tetrahydrofuran (872:110 :18), the flow rate is 1.2ml / min, the detection wavelength is 220nm, and the column temperature is 30°C.
[0057] Weigh various impurity reference substances, add mobile phase to dissolve and dilute to prepare about 100μg / ml of each impurity stock solution.
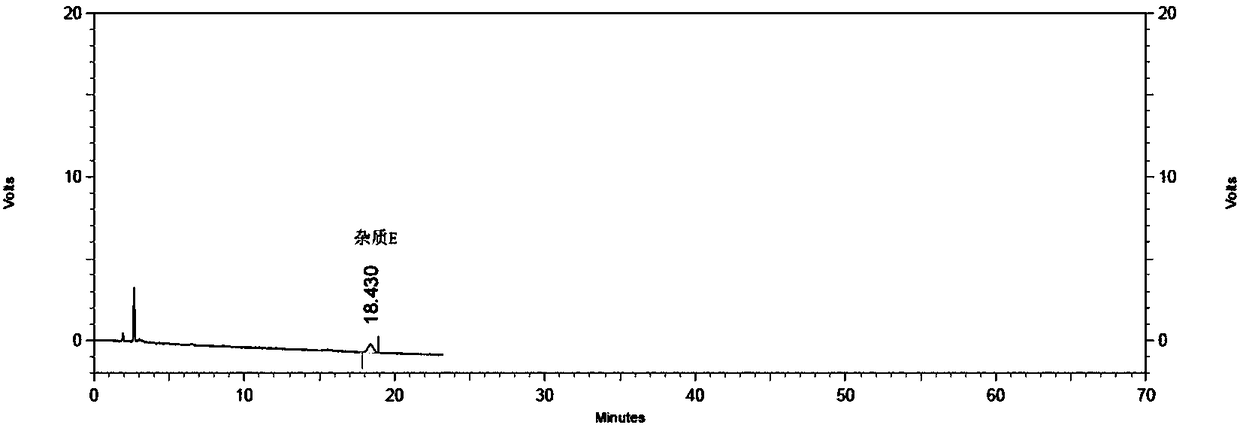
[0058] Mesalazine solution containing impurities A, B, C, D, F, O, P, and hydroquinone: Weigh an appropriate amount of mesalazine, and add impurities A, B, C, D, F, O, P, An appropriate amount of benzenediol stock solution, add mobile phase to dissolve and dilute to make containing mesalazine about 2mg / ml, containing impurities A and C about 0.2μg / ml, containing impurities B, D, F, O, P, p-benzene Diphenols are about 1μg / ml solution, which is obtained.
[0059] Impur...
Embodiment 3
[0067] Using a phenyl-bonded silica gel column, the mobile phase is the aqueous phase (4.43g potassium dihydrogen phosphate and 1.63g sodium octane sulfonate, dissolved in 870ml of water, add phosphoric acid to adjust the pH to 2.15)-methanol-acetonitrile-tetrahydrofuran (870 :108:4:18), the flow rate is 1.2ml / min, the detection wavelength is 220nm, and the column temperature is 25°C.
[0068] Weigh various impurity reference substances, add mobile phase to dissolve and dilute to prepare about 100μg / ml of each impurity stock solution.
[0069] Mesalazine solution containing impurities A, B, C, D, F, O, P, and hydroquinone: Weigh an appropriate amount of mesalamine, add impurities A, B, C, D, F, O, P, An appropriate amount of benzenediol stock solution, add mobile phase to dissolve and dilute to make containing mesalazine about 2mg / ml, containing impurities A and C about 0.2μg / ml, containing impurities B, D, F, O, P, p-benzene Diphenols are about 1μg / ml solution, which is obtained....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com