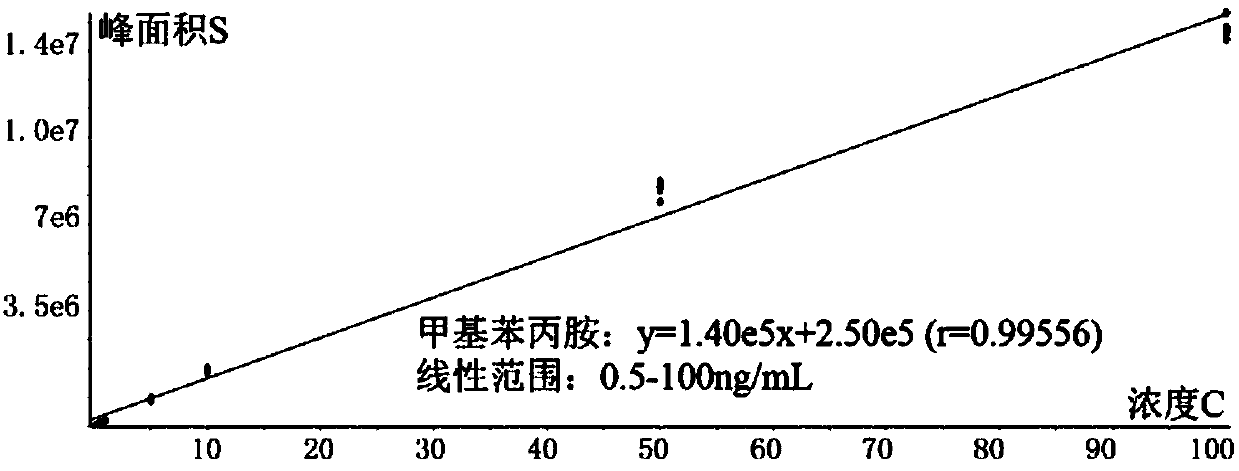
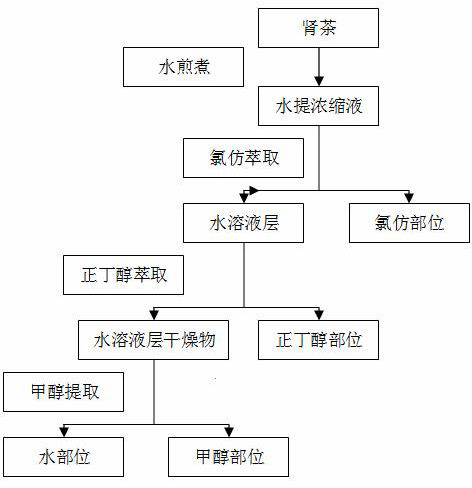
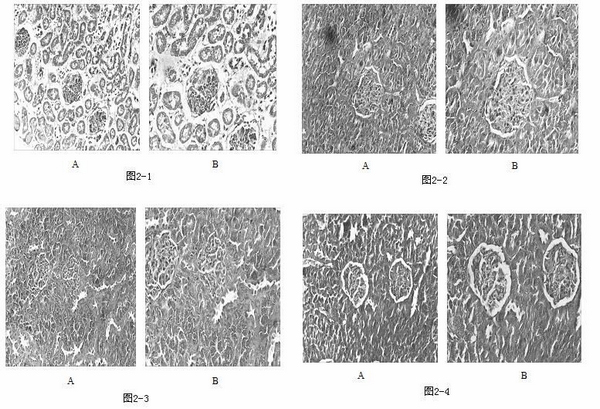
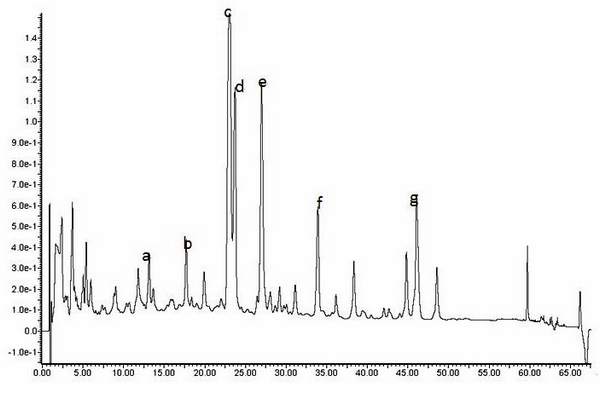
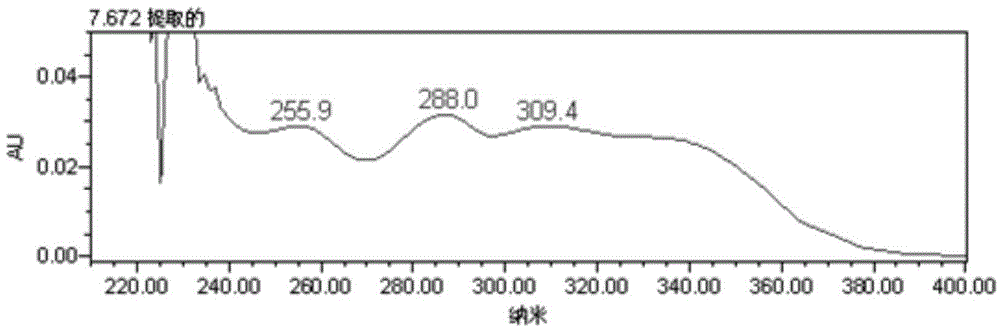
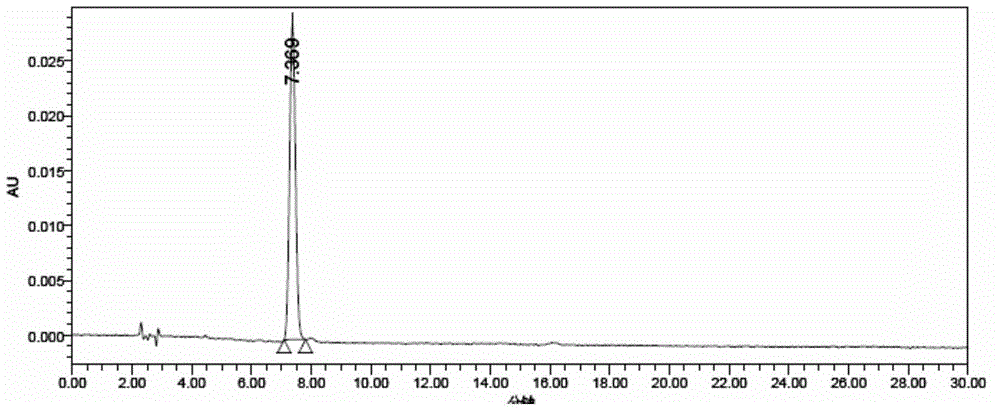
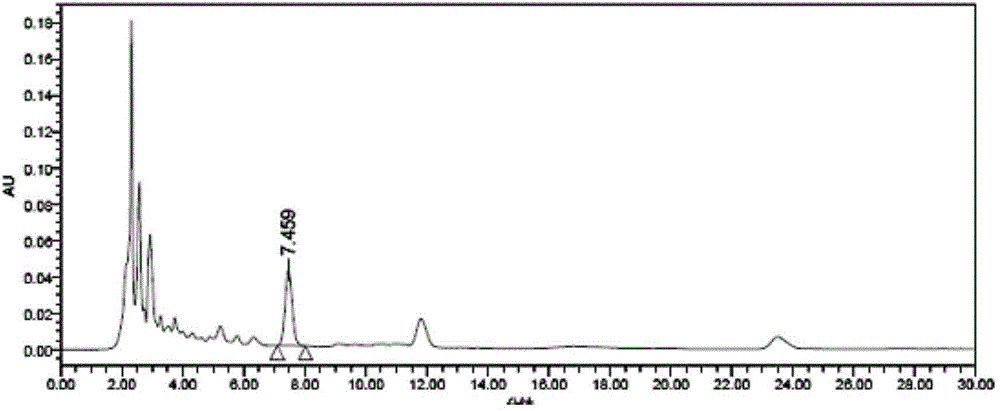
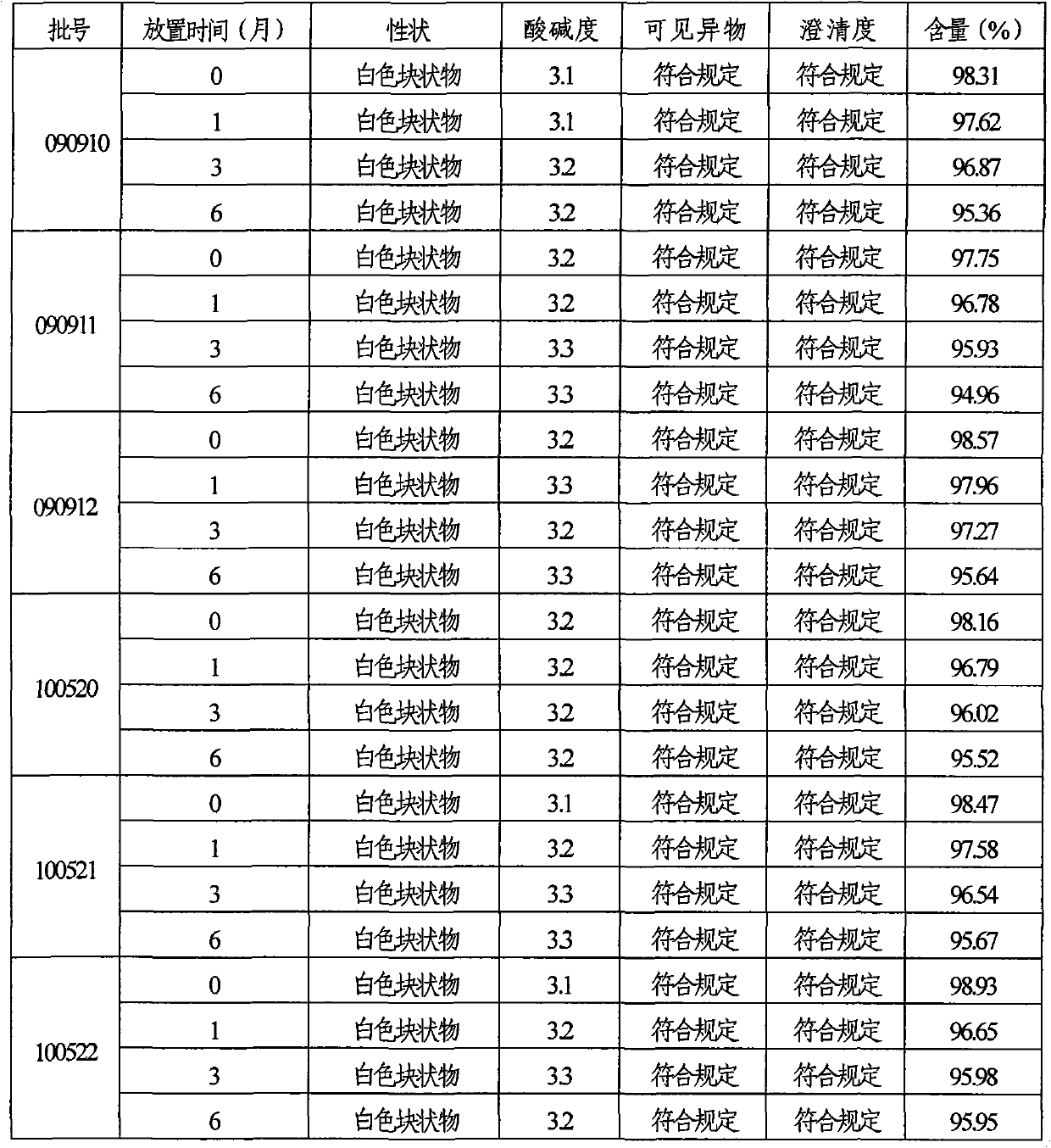
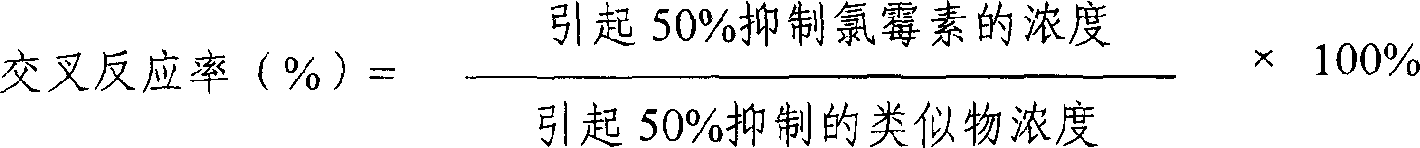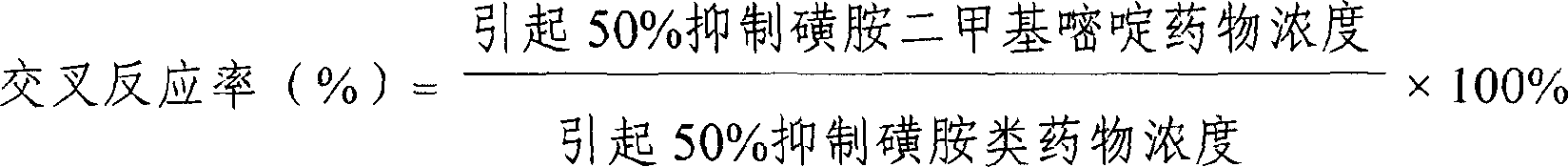Patents
Literature
142 results about "Drug standards" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug reference Standards are highly characterized physical specimens used in testing by pharmaceutical and related industries to help ensure the identity, strength, quality, and purity of medicines (drugs, biologics, and excipients), dietary supplements, and food ingredients.
Kit for detecting anti-psychosis drugs in serum and plasma by liquid chromatography tandem mass spectrometry method and application thereof
InactiveCN109085263AReduce matrix effectThe test result is accurateComponent separationPsychosis drug9-Hydroxyrisperidone
The invention provides a kit for detecting anti-psychosis drugs in a serum and plasma by a liquid chromatography tandem mass spectrometry method. The kit comprises drug standards: amsulpiride, aripiprazole, dehydroaripiprazole, chlorpromazine, clozapine, n-desmethylclozapine, risperidone, 9-hydroxyrisperidone, quetiapine, olanzapine, ziprasidone; drug internal standard compounds: amplepiride-d5, aripiprazole-d8, chlorpromazine-d3, clozapine-d8, risperidone-d4, 9-hydroxyrisperidone-d4, quetiapine-d8, olanzapine-d8, ziprasidone-d8; drug extraction compounds: methanol solution 60% (volume ratio),acetonitrile solution 20%, isopropanol solution 10%, purified water 10%; negative plasma; and a diluent: 50% aqueous methanol solution. The kit can be used to simultaneously detect anti-psychosis drugs and active metabolites thereof, the detection time is short and the flux is large.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
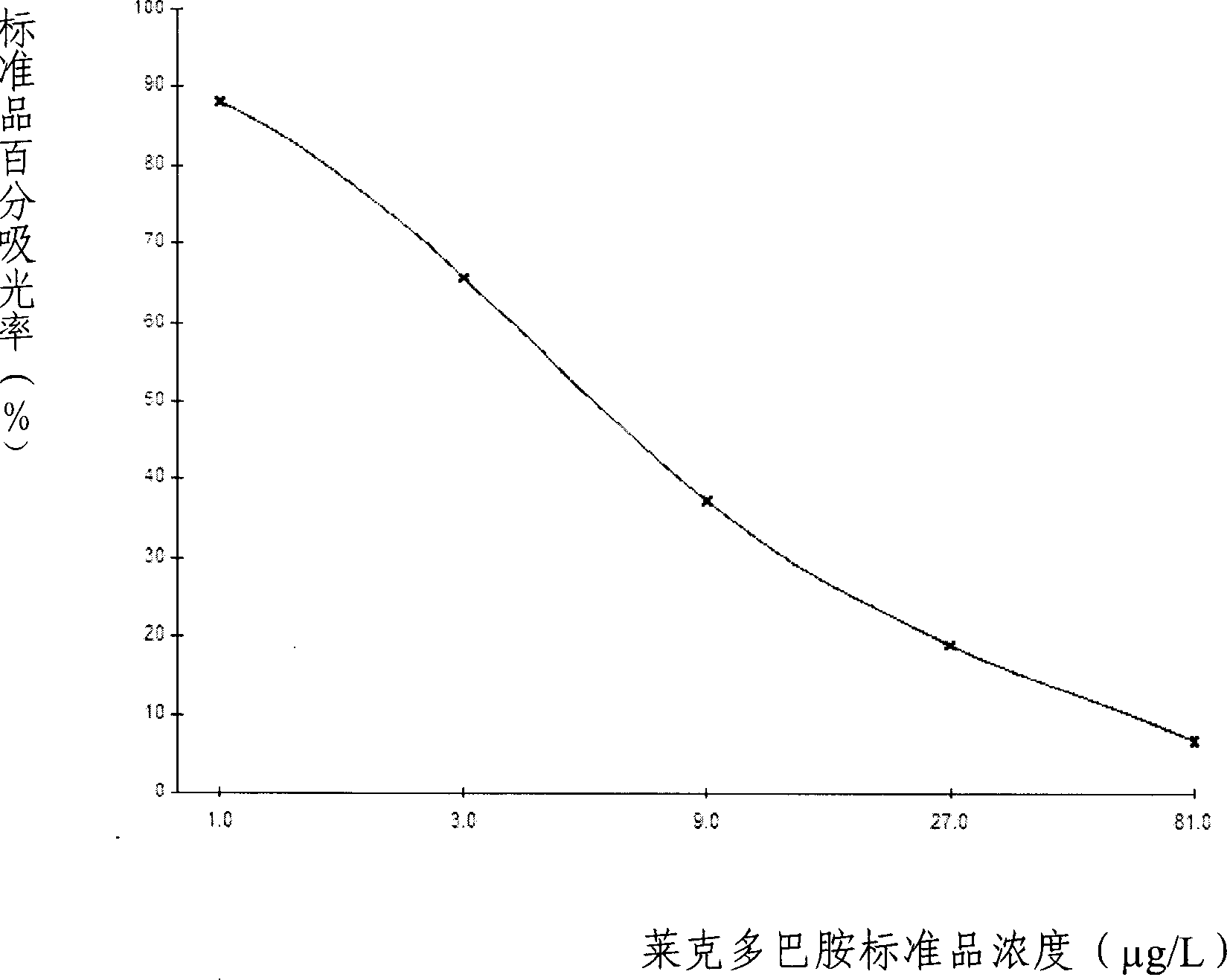
ELISA kit for detecting ractopamine in animal derived food
The invention discloses an enzyme immune agent box for detecting lake drugs of animal foodstuff; it also provides a method for using the agent to detect the lake drugs residue. The agent box comprises: enzyme mark plate which coats lake drugs antigen or antibody, lake drugs mouse monoclonal antibody or polyclonal antibody working solution, enzyme mark antibody or enzyme mark lake drugs antigen, lake drugs standard solution, base material color developing solution, compression cleaning liquid, ending solution and compression twin solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
Xinjiang chamomile planting technique
The invention belongs to the technical field of traditional Chinese medicine, relating to a Xinjiang chamomile planting technique. The planting technique provided by the invention solves the technical problems in chamomile seeding, staged fertilization, field management, harvesting, processing and the like, and realizes standardized planting of chamomile. Chamomile products planted by the technique provided by the invention meet the drug standard of ministry of public health of China, part of Uygur medicine, 1998, and the yield per mu of chamomile dried flowers can reach 60-100 kilograms.
Owner:XINJIANG MEDICAL UNIV
Quality test method of Dianxianning tablets
ActiveCN101732515AImprove quality control standardsHigh level of quality standardsWeighing by removing componentNervous disorderValeriana jatamansiPhysical chemical
The invention relates to a quality test method of Dianxianning tablets (WS3-B-2823-97) in volume 4 of health ministry drug standard of People's Republic of China (traditional Chinese medicine set prescription preparation). The quality test method of the Dianxianning tablets improves the quality control standard of the Dianxianning tablets, establishes a test method of volatile ether extract in the preparation, cancels a physical-chemical reaction identification method with poor specificity, improves a thin-layer chromatography identification method of valeriana jatamansi jones, enables the method to be more accurate, increases the thin-layer chromatography identification methods of uncaria and acorus gramineus and ensures that the compound preparation has higher quality standard level.
Owner:KUNMING CHINESE MEDICINE FACTORY
Preparation method of high-purity naringenin
The invention provides a preparation method of naringenin. The preparation method comprises the following steps: (1) preparing solution of naringin in lower alcohol or lower ketone solvent, leading the naringin in the prepared solution to be subjected to acid hydrolysis in an acidic condition to obtain naringenin, distilling under reduced pressure to remove solvent to obtain a naringenin crude product; (2) dissolving the naringenin crude product in absolute ethanol or absolute methanol, adding active carbon, stirring to decolor; and (3) filtering the active carbon, separating and purifying the prepared solution to obtain the naringenin. The preparation method has the advantages of few reaction steps, less used solvent, less emission of the 'three wastes', mild reaction condition and complete reaction and easy operation, is suitable for industrial production, and has higher practical value; and the prepared product has more stable quality, the purity is higher than 99 percent, which is higher than the drug standard.
Owner:HENAN TOPFOND PHARMA
ELISA kit for detecting chloramphenicols in animal derived food
ActiveCN1766629AQuick checkLow pre-processing requirementsBiological testingSite monitoringElisa kit
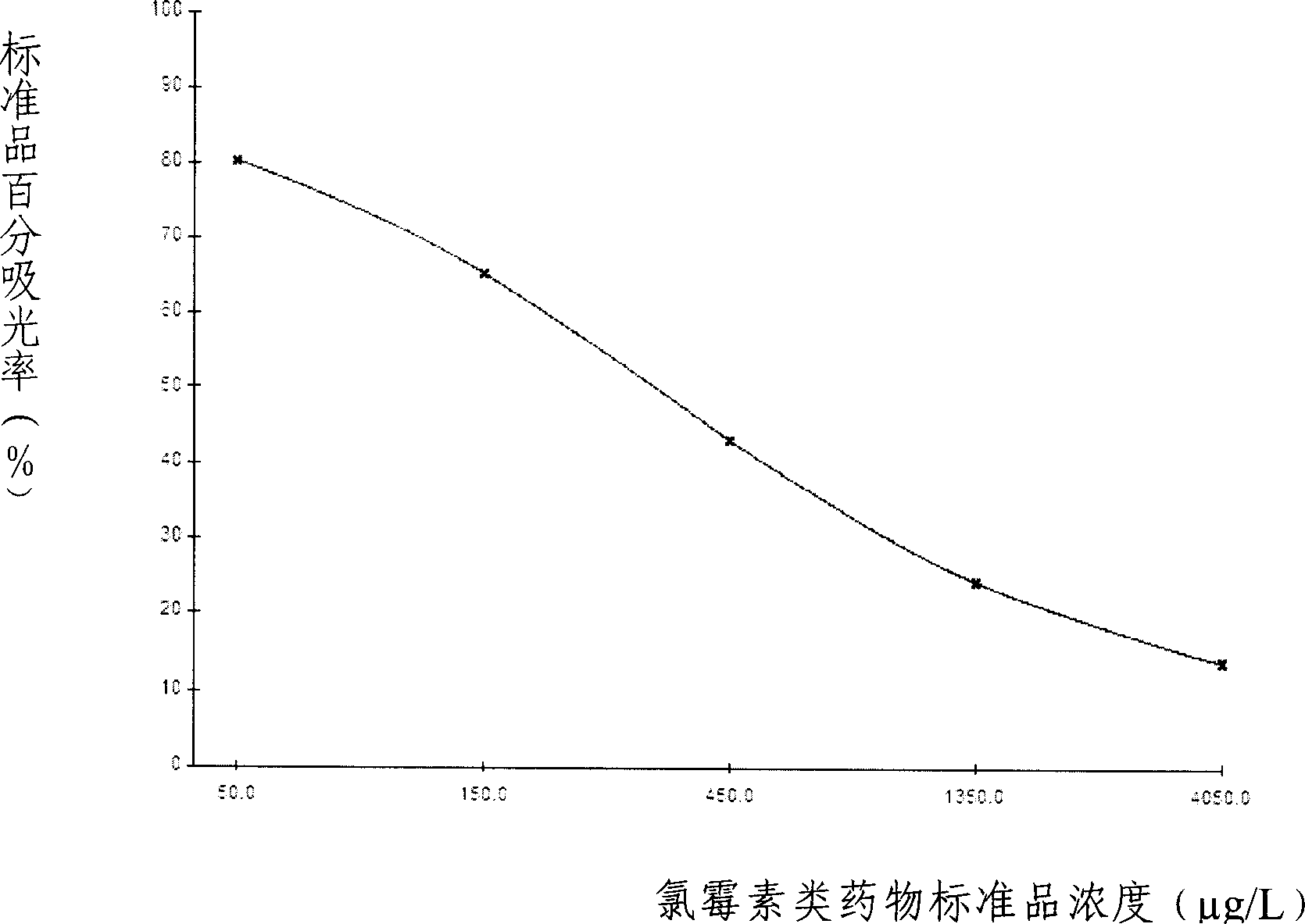
The invention provides an enzyme immune agent box for detecting chloromycetin drugs of animal foodstuff which comprises: enzyme mark plate which coats chloromycetin drugs antigen or antibody, chloromycetin drugs mouse monoclonal antibody compression solution, chloromycetin drugs standard solution, enzyme mark material, base material color developing solution, compression cleaning liquid, ending solution and compression twin solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
ELISA kit for detecting sulfanilamides residue in animal derived food
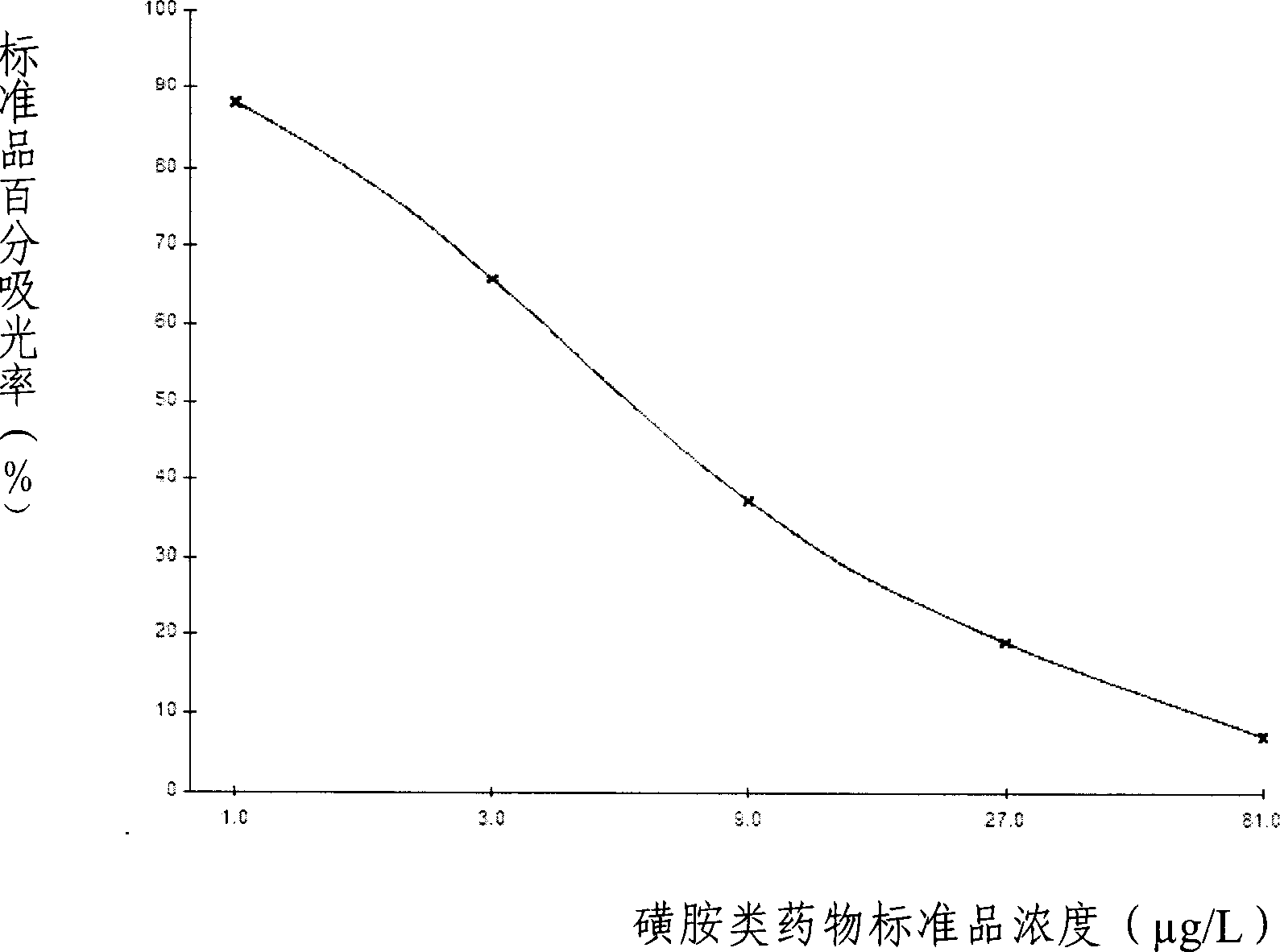
The invention provides an enzyme immune agent box for detecting sulpha drugs of animal organization, which uses enzyme immune method to detect the preprocessed animal organization, honey, urine, milk. The enzyme immune agent box comprises: enzyme mark plate which coats sulpha drugs antigen or antibody, sulpha drugs mouse monoclonal antibody working solution, enzyme mark antibody or sulpha drugs antigen solution, sulpha drugs standard solution, base material color developing solution, compression cleaning liquid, ending solution and compression extracting solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
Kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085264APharmacologically activeInterpret blood levelsComponent separationSertralineTandem mass spectrometry
The invention provides a kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry. The kit comprises drug standard substances, drug internal standardization compounds, drug extraction compositions, negative plasma and a diluent. The drug standard substances comprise amfebutamone, oxybupropion, citalopram, Escitalopram, venlafaxine, O-desmethylvenlafaxine, duloxetine, fluoxetine, norfloxetine, fluvoxamine, mirtazapine, paroxetine, sertraline and trazodone. The drug internal standardization compounds comprise amfebutamone-d9, oxybupropion-d6,citalopram-d6, venlafaxine-d6, O-desmethylvenlafaxine-d6, duloxetine-d3, fluoxetine-d6, norfloxetine-d6, fluvoxamine-d4, mirtazapine-d3, paroxetine-d6, sertraline-d3 and trazodone-d6. The drug extraction compositions comprise, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropyl alcohol solution and 10% of purified water. The diluent comprises 50 % of methanol waterfluid. The kit can be used for simultaneous detection of the anti-depressant drugs and active metabolites, the detection time is short, and flux is high.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Synthesis method of middle-molecular-weight hydroxyethyl starch
The invention provides a synthesis method of middle-molecular-weight hydroxyethyl starch. The synthesis method comprises the following steps: carrying out hydroxyethyl substitution reaction on waxy corn starch hydrolyzate and a hydroxyethyl substituting agent under the conditions that water is used as a solvent and sodium hydroxide is taken as a catalyst; and carrying out ultrafiltration interception membrane separation and activated carbon decoloration, filtering, and carrying out spray drying on filtrate so as to obtain the white powdery middle-molecular-weight hydroxyethyl starch product. According to the invention, an adopted aqueous phase synthesis method has the advantages that an ideal molar degree of substitution (MS) of hydroxyethyl and an ideal substitution position ratio (C2:C6) are conveniently controlled in the reaction, and the byproducts of the reaction are easy to separate; and under the condition of the aqueous phase synthesis method, the reaction is a room temperature reaction, reactants and the catalyst are completely dissolved in the reaction system, the substitution positions are uniformly distributed in the product, the reaction byproducts such as sodium chloride, chlorohydrin, cyclochloroethane, glycol and the like can be easily removed once through a membrane separation technology, the obtained product has proper MS and a ratio of C2:C6, and the obtained product quality can reach or be superior to the existing national drug standard.
Owner:WUHAN HUST LIFE SCI & TECH
Quality standard and test method of hoove pill and preparation threrewith
The invention discloses an ascites pill and a quality control method for the preparation of the ascites pill, wherein the preparation is provided with a plurality of formulations by adding conventional accessories according to the ascites pill preparation formula in the second volume of traditional Chinese medicine set prescriptions preparations of Drug Standards of Ministry of Health of the People's Republic of China. The quality control method which comprises a content determination or content identification or one of the content determination and the content identification increases the thin layer identification of euphorbia Gansui Root, jujube oleanolic acid and elecampane based on the original quality standards, revises the thin layer identification of melanterite and builds the content determination for the green copperas (FeSO4 7H2O) and improves the controllability of the product quality.
Owner:HANDAN PHARMA
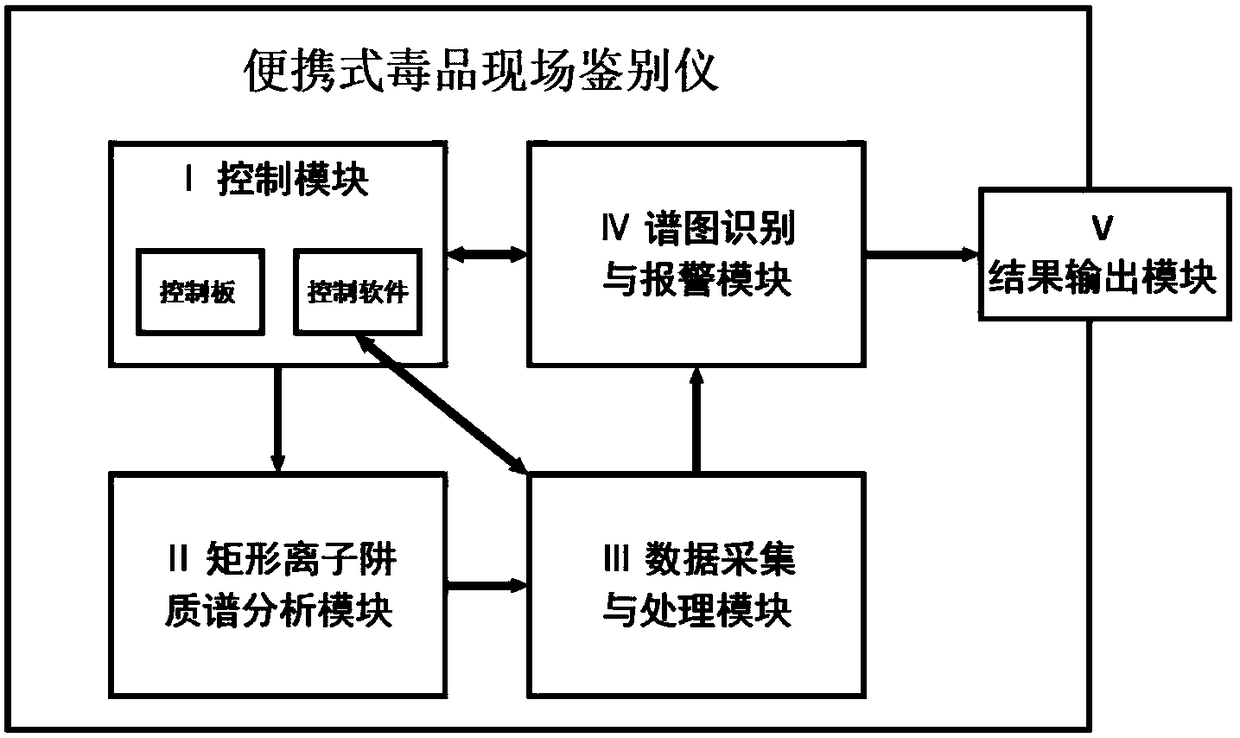
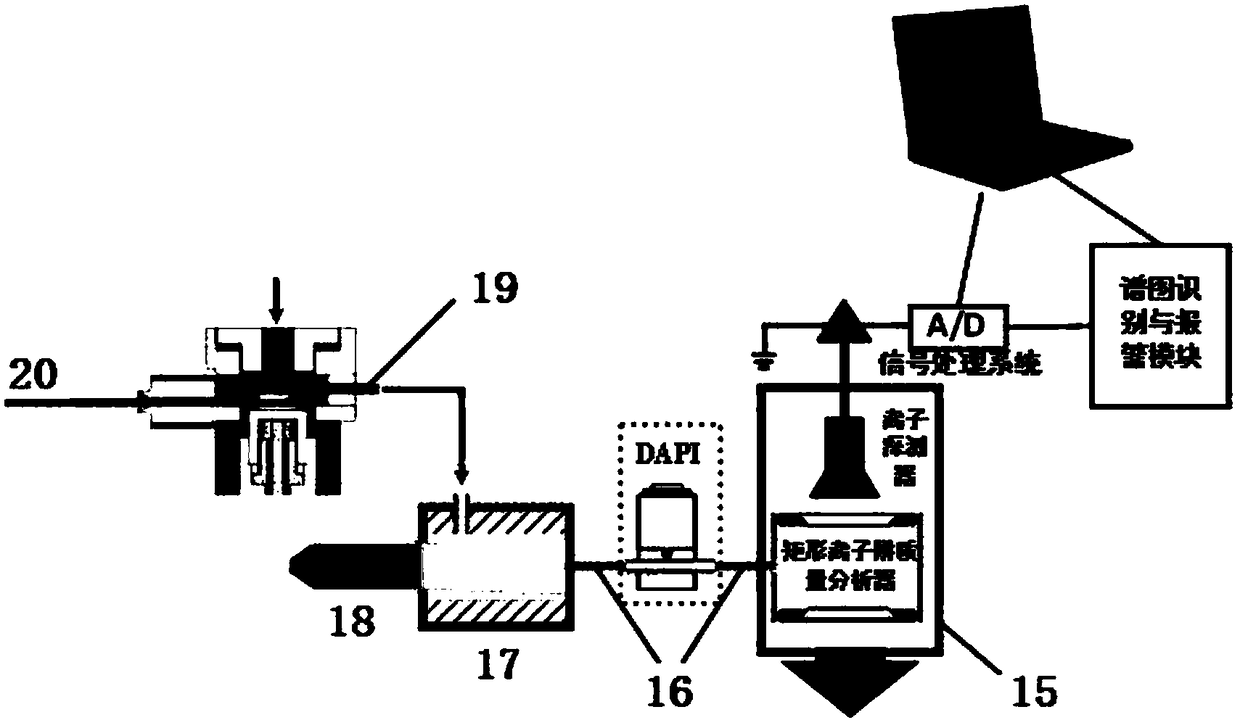
Portable drug field identification equipment and method
InactiveCN108072692AHigh sensitivityHigh qualitative detection accuracyMaterial analysis by electric/magnetic meansData acquisitionEngineering
The invention relates to portable drug field identification equipment and method. The equipment comprises a control module, a rectangular ion trap mass spectrome analysis module, a data collection andprocessing module, a spectrogram recognition and warning module and a result output module, wherein a drug test specimen or a practical sample generates current signals after passing through identification equipment; the current signals are converted into voltage signals; an inside clock outputs time signals; voltage signals are converted into digital signals; time signals and digital signals areextracted; normalization and base line correction processing are performed; if a sample is a drug standard sample, a standard spectrum base is generated; if the sample is a practical sample, a samplespectrogram is generated; the sample spectrogram and the standard spectrum base are subjected to feature comparison. The equipment and the method have the advantages that the size is small; the weight is light; complicated pre-treatment is not needed; liquid samples or solid samples are directly subjected to feeding sample detection; the automatic recognition, warning and printing on the detection result can be realized; the single sample detection and warning time is shorter than 2s; the equipment and the method are suitable for drug field investigation and seizing and identification; wide application prospects are realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for producing L-tyrosine through enzyme method
The invention discloses a method for producing L-tyrosine through an enzyme method. The method comprises the following steps of adopting a fermentation method to produce pyruvic acid, adding beta-tyrosinase, phenol and NH4Cl at the fermentation anaphase of the pyruvic acid, converting the generated pyruvic acid into the L-tyrosine, and obtaining fermentation liquor containing the L-tyrosine; heating the fermentation liquor containing the L-tyrosine to be 70 DEG C to 90 DEG C, and acidifying until L-tyrosine crystals are completely dissolved; adding activated carbon to decolor, after decoloring, filtering by utilizing a ceramic membrane, removing bacteria, albumen and the activated carton, carrying out secondary filtering on obtained clear liquid through an acid-resisting liquid cartridge filter; adjusting the pH of the secondary filtered clear liquid to be 5.0 to 7.0, cooling to crystallize, centrifuging, and obtaining the L-tyrosine. According to the method for producing the L-tyrosine through the enzyme method provided by the invention, the process is simplified, the production efficiency is improved, the obtained L-tyrosine is high in purity and yield, the purity of the obtained tyrosine is remarkably improved and reaches to 98.0 percent or above compared with a traditional extraction method, and various tyrosine drug standards are met.
Owner:SHANDONG YANGCHENG BIOLOGY TECH CO LTD
Quality detection method for Jinhuaxiaocuo pills
ActiveCN101732463AImprove quality control standardsHigh level of quality standardsComponent separationDigestive systemChemical reactionAdditive ingredient
The invention relates to a quality detection method for Jinhuaxiaocuo pills (WS3-B-2169-96) in the eleventh volume of Drug Standard of Ministry of Public Health of the Peoples Republic of China (Chinese patent medicaments). Through the quality detection method for the Jinhuaxiaocuo pills, the quality control standard for the Jinhuaxiaocuo pills is improved, a method for detecting the content of a main ingredient in a preparation is established, physical and chemical reaction identification with poor specificity is eliminated, and thin layer chromatography identification methods for geniposide and berberine hydrochloride are improved to have the advantages of simplness and clear characteristic spots, thin layer chromatography identification methods for rhubarb, golden thread, amur corktree bark and baical skullcap root in the Jinhuaxiaocuo pills are increased, and the high quality standard level of the compound preparation is guaranteed.
Owner:KUNMING CHINESE MEDICINE FACTORY
Preparation method of propofol fat emulsion injection
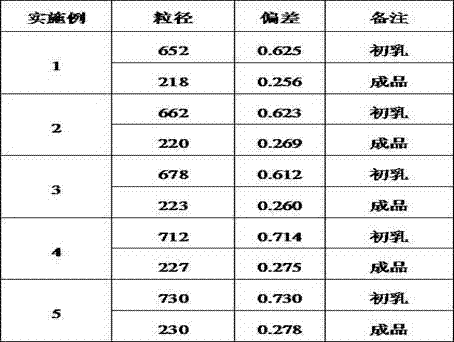
ActiveCN104490780AImprove the shortcomings of excessive particle size deviationMeet particle size requirementsHydroxy compound active ingredientsPharmaceutical product form changeSide effectOil phase
The invention discloses a preparation method of a propofol fat emulsion injection. The preparation method comprises the following steps: uniformly mixing soybean oil, lecithin and oleic acid and adding propofol; spraying an oil phase into a water phase under the protection of nitrogen gas to prepare emulsion; shearing the emulsion to obtain primary emulsion; homogenizing the primary emulsion under the pressure of 10000psi-20000psi to prepare an emulsion semi-finished product; and filtering, inflating nitrogen and sterilizing to prepare the propofol fat emulsion injection. According to the preparation method, the grain diameter uniformity is enhanced and the disadvantage that the grain diameter deviation of the primary emulsion is too great is improved; the stability of finished-product emulsion is improved; the average grain diameter of the detected emulsion is 210nm-230nm and the grain diameter deviation is 0.20-0.30; the requirements on the grain diameters by the fat emulsion injection are met; the emulsion grains with the size being more than 1 micron are not detected, and the grain diameters are obviously better than the standards that the content of the emulsion grains with the size being 1 micron in emulsion large grains of national drug standards is not more than 3%; the physicochemical properties are stable, the toxic side effect is low, the pains caused by injection are reduced, and the compliance of using drugs by patients is increased, so that the application prospect is very good.
Owner:HEBEI YIPIN PHARMA
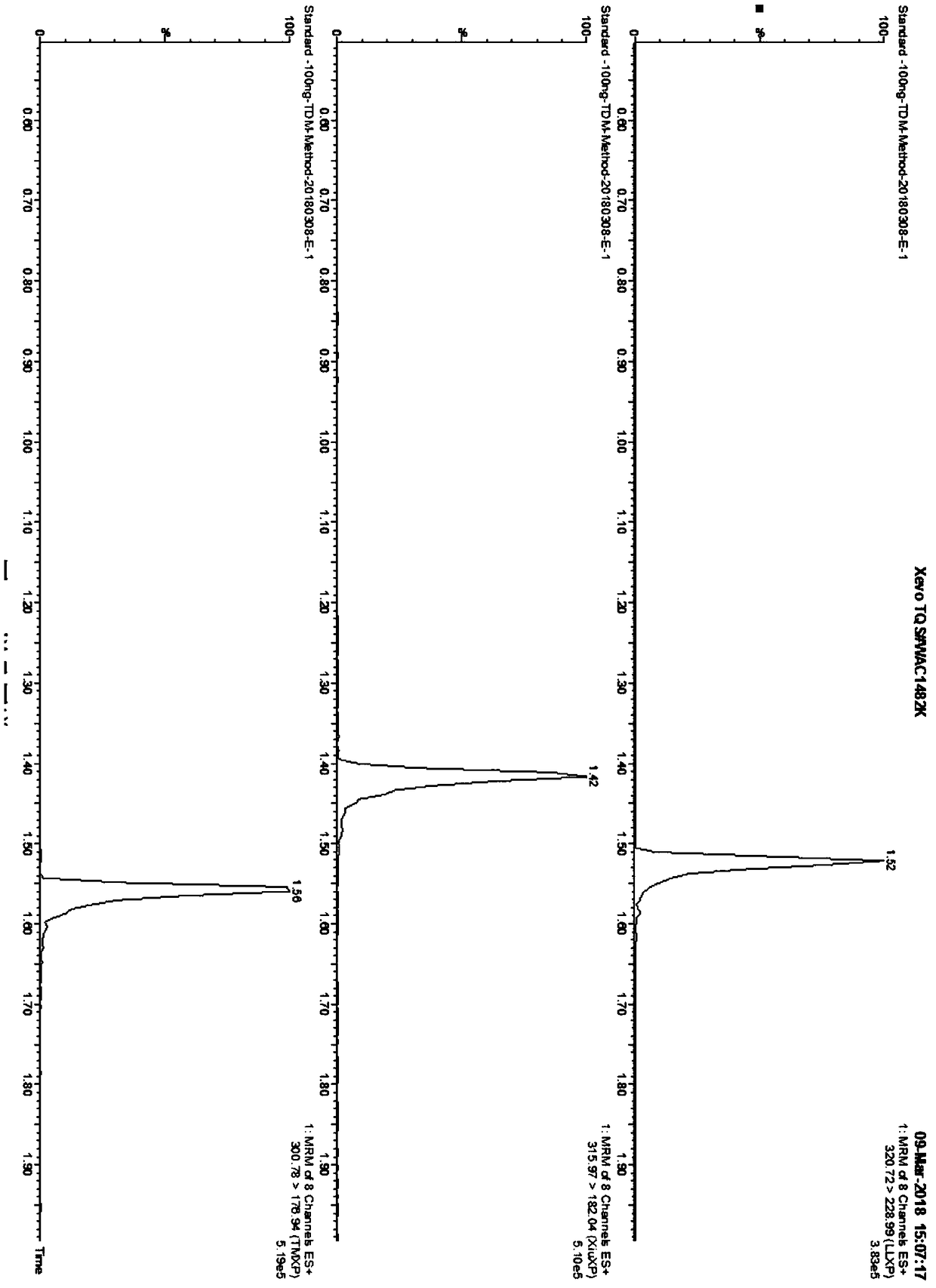
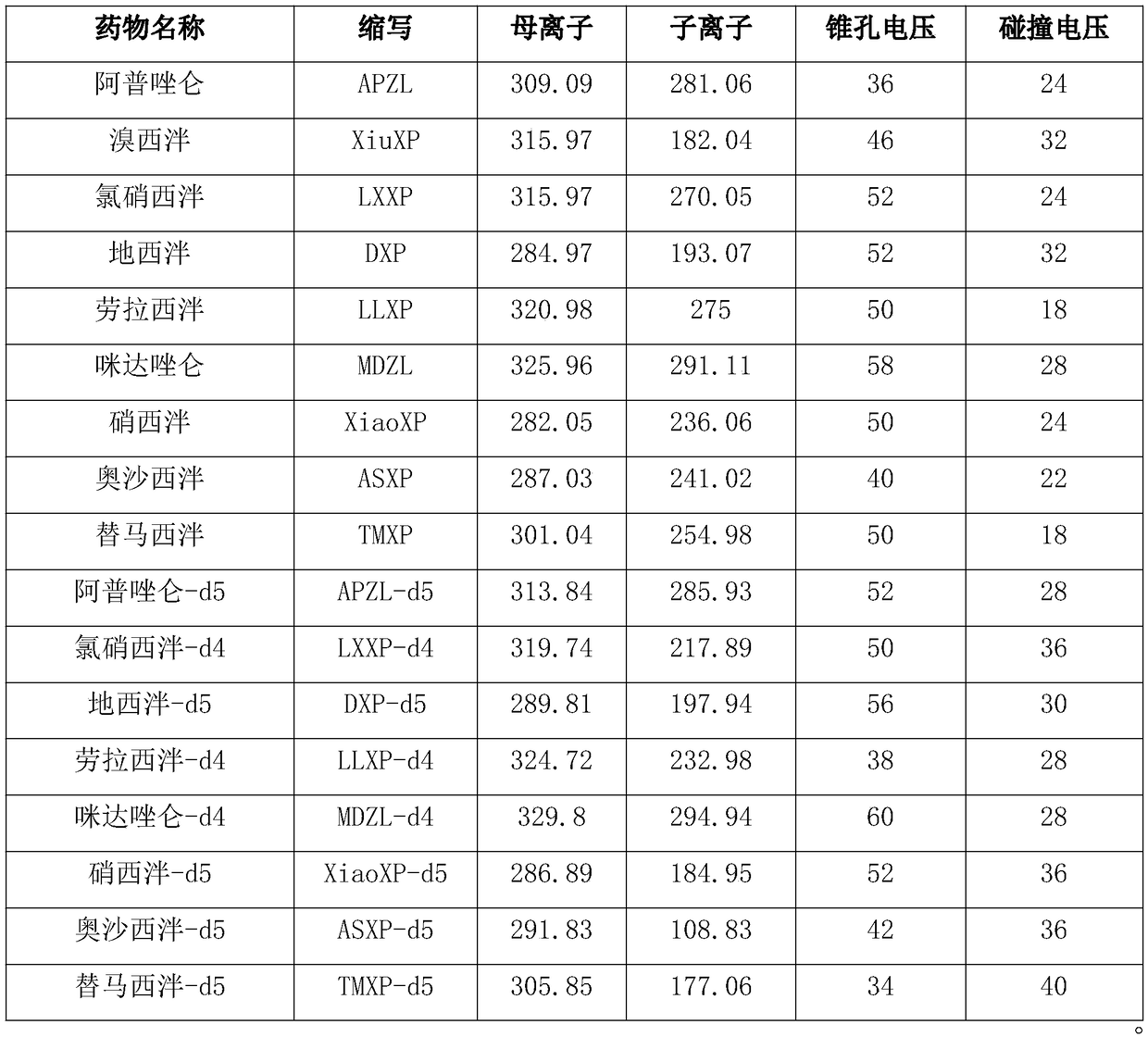
Kit for determining antianxiety/hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085265AReduce matrix effectThe test result is accurateComponent separationBromazepamTandem mass spectrometry
The invention provides a kit for determining antianxiety / hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry. The kit comprises the following constituents: drug standards comprising bromazepam, clonazepam, diazepam, lorazepam, midazolam, nitrazepam, oxazepam and Temazepam; drug internal standard compounds comprising alprazolam-d5, clonazepam-d4, diazepam-d5, lorazepam-d4, midazolam-d4, nitrazepam-d5, oxazepam-d5 and Temazepam-d5; drug extraction compositions comprising, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropanol solution and 10% of purified water; negative plasma; and diluent: 50% of carbinol water solution. The kit can be used for simultaneously determining antianxiety / hypnotic type drugs and active metabolites thereof, and has the advantages of short determination time and high flux.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
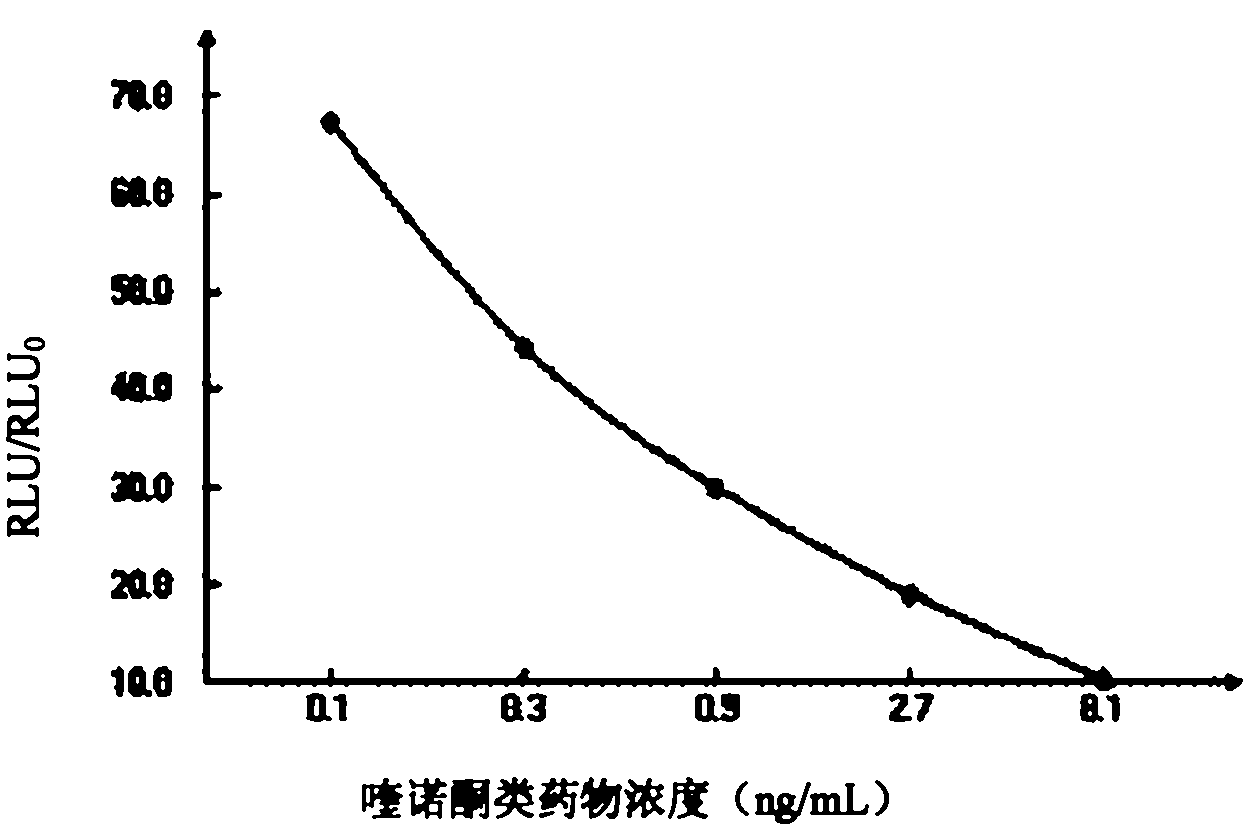
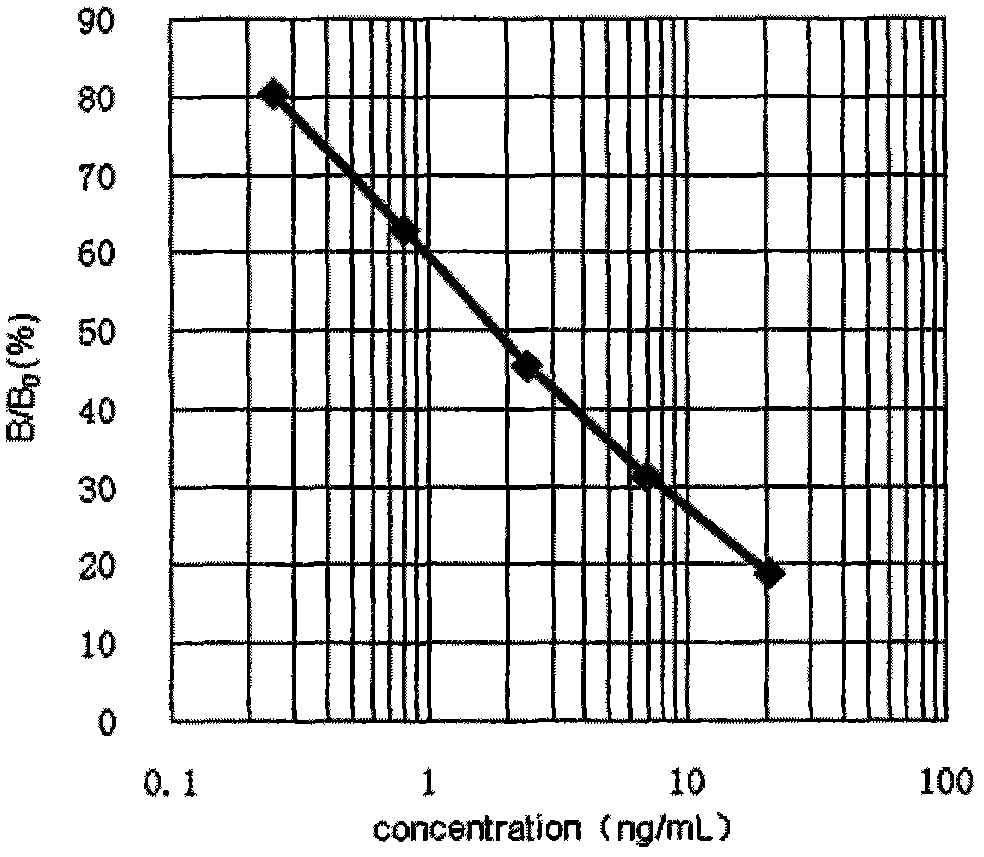
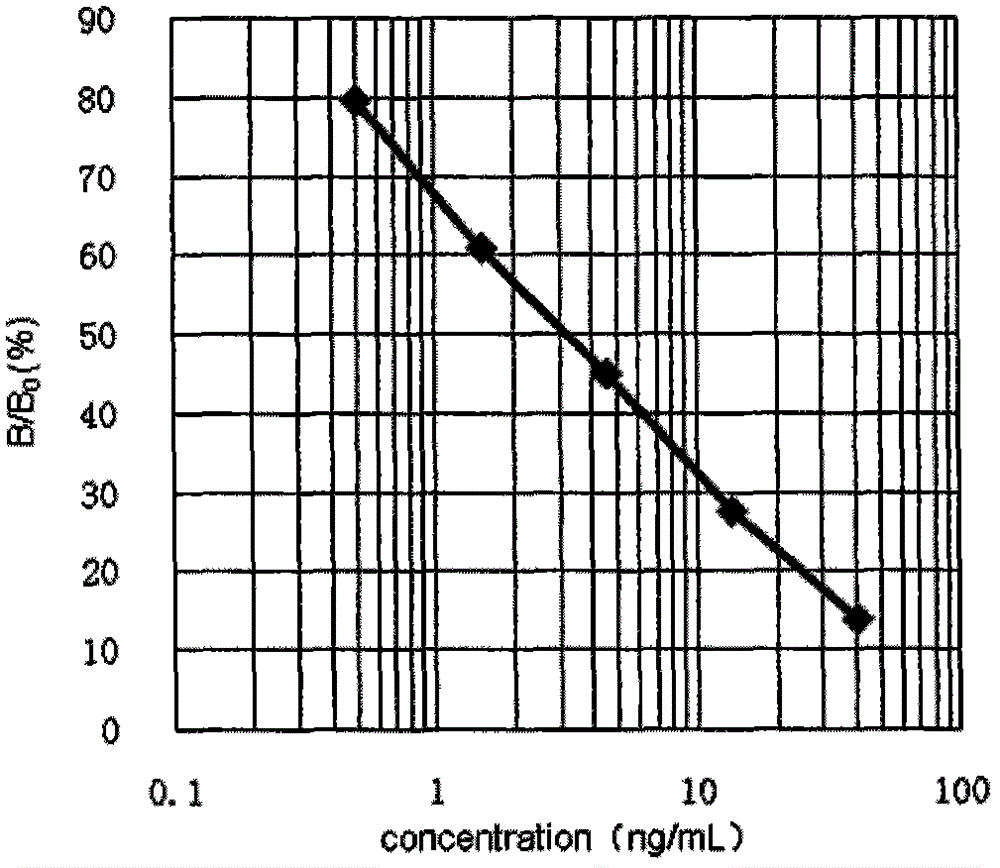
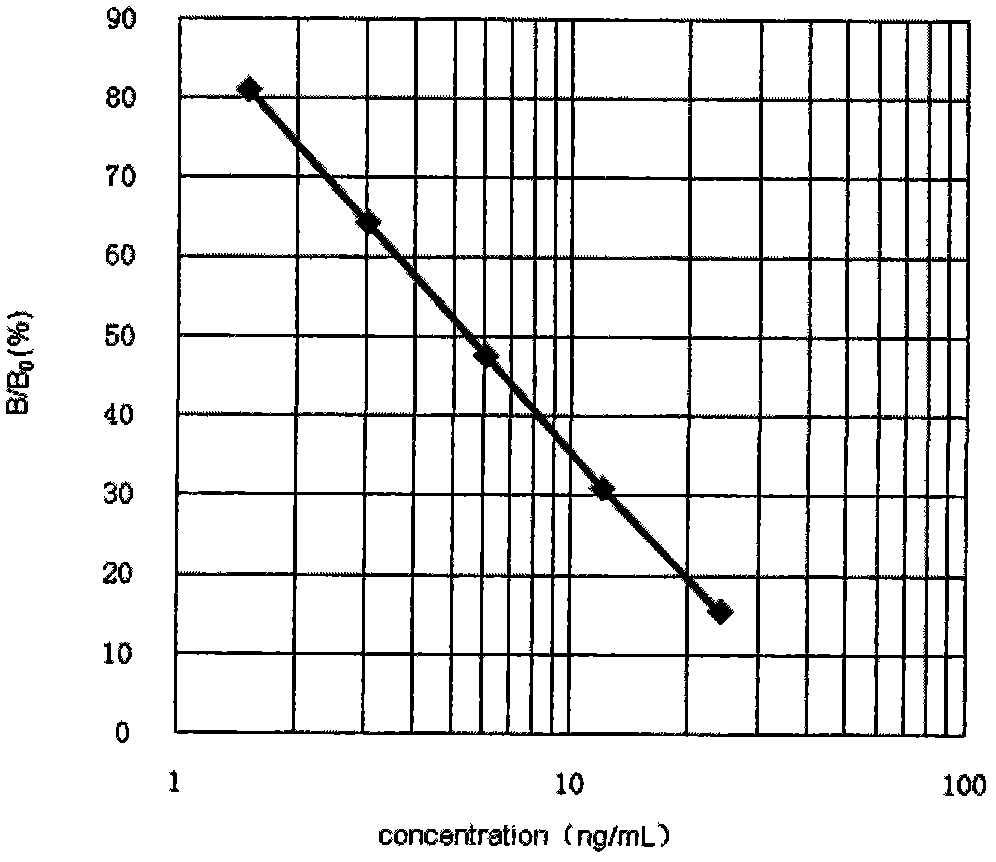
Quinolone drug chemiluminescence enzyme-linked immunodetection kit
The present invention discloses a quinolone drug chemiluminescence enzyme-linked immunodetection kit, which comprises a kit body, an enzyme label plate placed inside the kit body, and reagents placed inside the kit body, and is characterized in that every hole of the enzyme label plate is coated with coating antigen, the coating antigen is a norfloxacin derivative and carrier protein conjugate, and the reagents comprise quinolone drug monoclonal antibody, horseradish peroxidase-labeled goat anti-mouse antibody, a series of quinolone drug standard solutions, a concentrated phosphate buffer, a concentrated washing solution and a chemiluminescence solution. The quinolone drug chemiluminescence enzyme-linked immunodetection kit has characteristics of high sensitivity, simple and rapid detection, high accuracy, and more drug detection types, provides a substantially reduced operation time compared to the conventional colorimetric ELISA method, and can be used for detection of residues of the 12 quinolone drugs in animal tissues (pork, chicken, pork liver and chicken liver), aquatic products (fish and shrimp) and honey.
Owner:BEIJING KWINBON BIOTECH
Traditional Chinese medicine ophthalmic preparation and preparation method thereof
InactiveCN101579363AHigh dissolution rateReduce graininessPowder deliverySenses disorderDiseaseClinical efficacy
The invention discloses a traditional Chinese medicine preparation, in particular a traditional Chinese medicine preparation for treating ophthalmic diseases and a preparation method thereof. The prepared preparation is powder, ointment or gelata and belongs to the field of traditional Chinese medicine. The preparation has the advantages that the prescription originates from a classic and famous transcription of the Drug Standard of Ministry of Public Health of the People's Republic of China, the preparation method induces an ultrafine grinding technology for the first time, the raw materials (especially minerals and animal medicines) have thorough grinding, small granularity and large specific area, active ingredients can be better exposed to be in a release condition, so the dissolution rate of the active ingredients is greatly enhanced, the biological availability is enhanced multiply and the general reaction of clinic curative effect is good; at the same time, the foreign body sensation is obviously relieved and granular sensation almost disappears when the preparation is used by a suffer, thereby the preparation is an ideal medicine for treating ophthalmic diseases, is deeply popularized with suffers and has wide market prospect.
Owner:BEIJING HERUN INNOVATION PHARMA TECH DEV
Drug testing enzyme linked immunosorbent assay kit and detection method thereof
The invention discloses an enzyme linked immunosorbent assay kit for detecting drugs in urine, saliva, blood and hairs in the technical field of the prohibited goods detection and analysis as well as a detection method of the drug testing enzyme linked immunosorbent assay kit. The kit comprises a drug standard substance solution, a drug specificity antibody, an elisa plate which is coated with the drug specificity antibody, a drug antigen enzyme marker, a color developing agent, concentrated washing liquor, a termination solution and a sample dilute solution. The enzyme linked immunosorbent assay kit has characteristics of high specificity, high sensitivity, high accuracy and the like and plays an important role in drug testing.
Owner:HANGZHOU UMOTOR BIOTECH CO LTD
Medical carbon dioxide absorbent soda lime and preparation method thereof
InactiveCN102258938AImprove brittlenessNot easy to dryDispersed particle separationAir quality improvementMedicineCo2 absorption
The invention discloses soda lime as a medical carbon dioxide absorbent and a preparation method thereof. The soda lime comprises the following components in percentage by weight: 75-85% of Ca(OH), 0.5-5% of NaOH, 0-3% of KOH, 0.1-1% of humectant, 0.5-5% of disintegrating agent, 0.005-1% of pH indication coloring agent and 10-20% of water. The soda lime prepared by the invention has better friability, is uneasy to generate fine power and dry, and the CO2 absorption rate meets the requirement of national drug standards.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Marketed drug information standardization method and device, server and storage medium
PendingCN111180087ACollect fast and comprehensiveNatural language data processingDrug referencesNetwork onEngineering
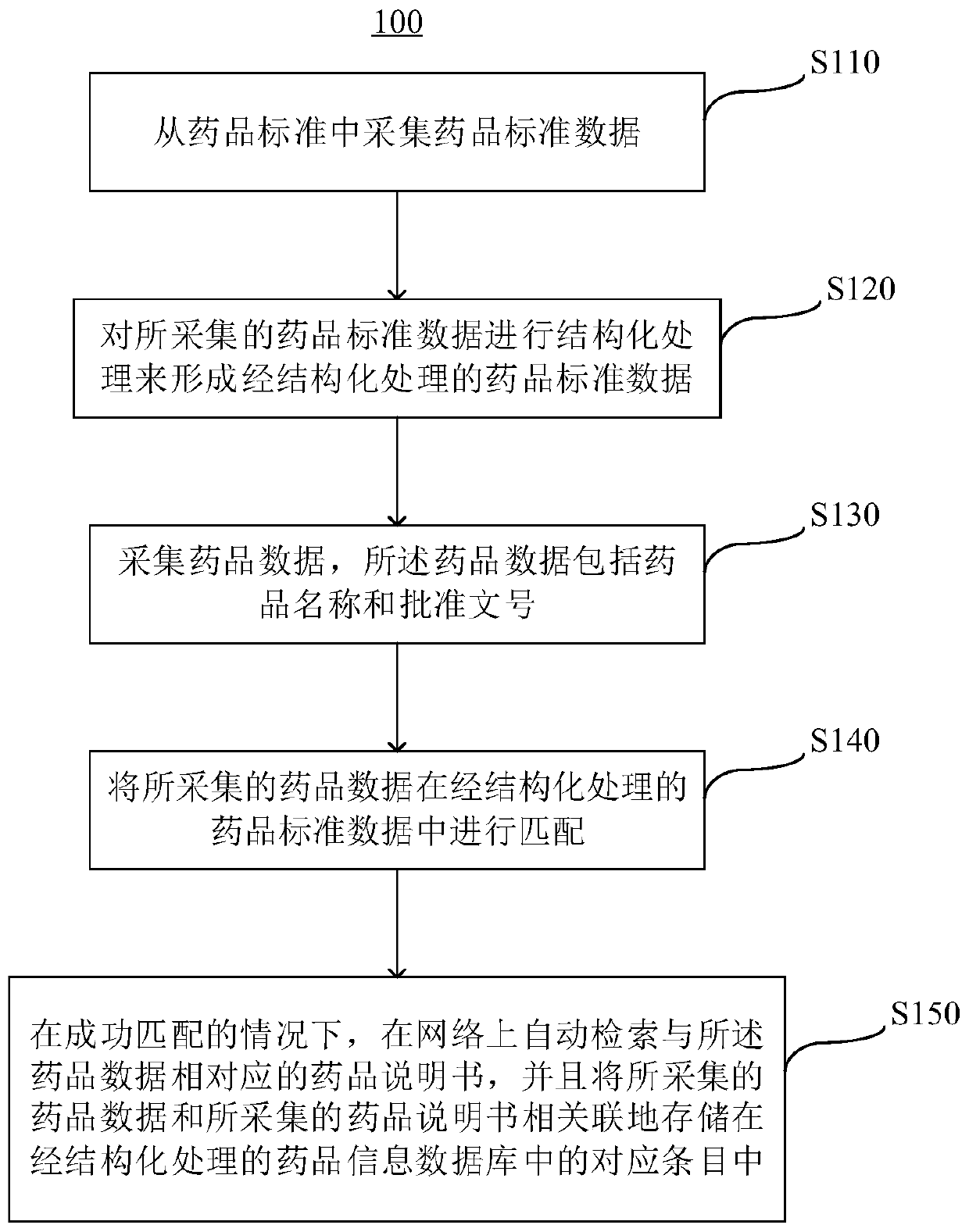
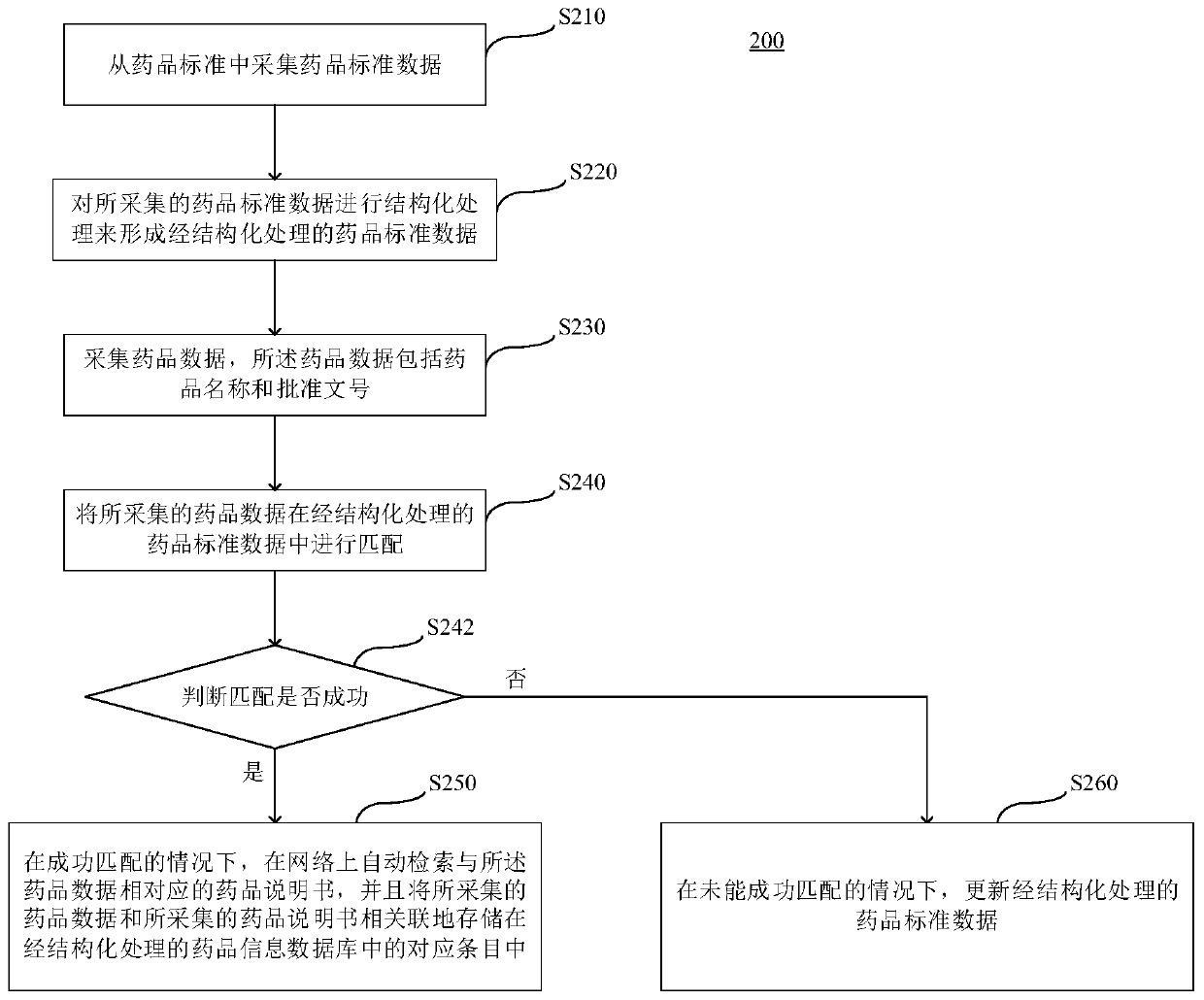
The embodiment of the invention relates to a marketed drug information standardization method and device, a server and a computer readable storage medium. The method comprises the following steps: collecting drug standard data from drug standards; carrying out structured processing on the collected drug standard data to form structured drug standard data; collecting drug data, wherein the drug data comprises a drug name and an approval document number; matching the acquired drug data in the structured drug standard data; and in the case of successful matching, automatically retrieving a drug specification corresponding to the drug data on a network, and storing the acquired drug data and the acquired drug specification in association in corresponding entries in a structured drug information database. Therefore, the invention provides the method for collecting and providing standardized marketed drug information more quickly and comprehensively.
Owner:INST OF INFORMATION ON TRADITIONAL CHINESE MEDICINE CACMS
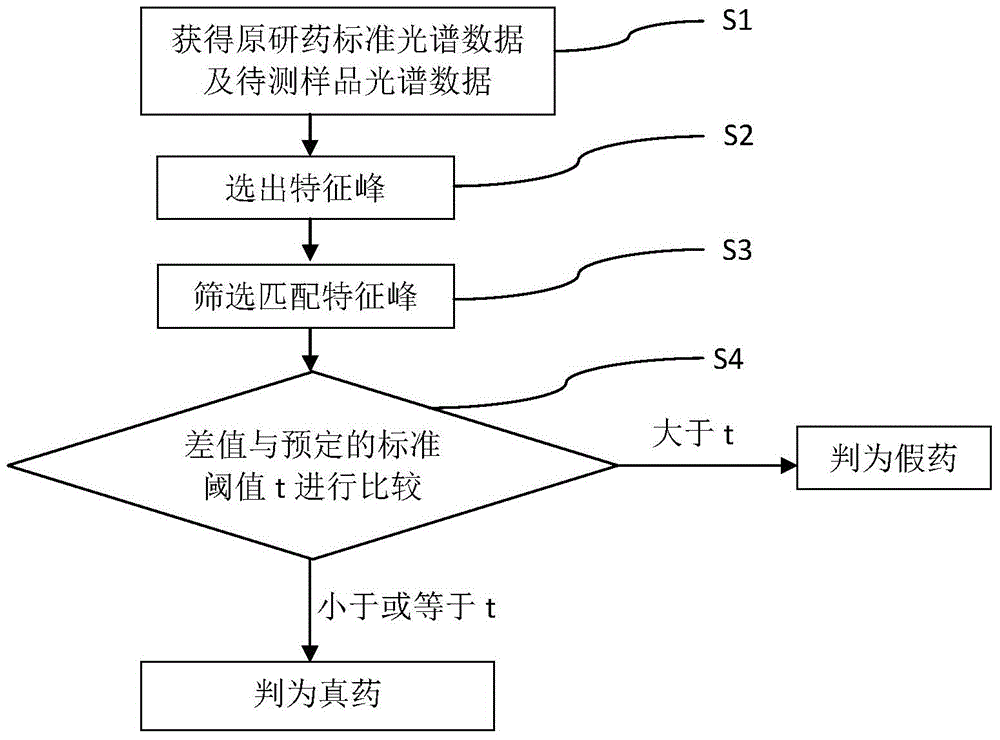
Method for detecting generic drug pretended to be reference listed drug
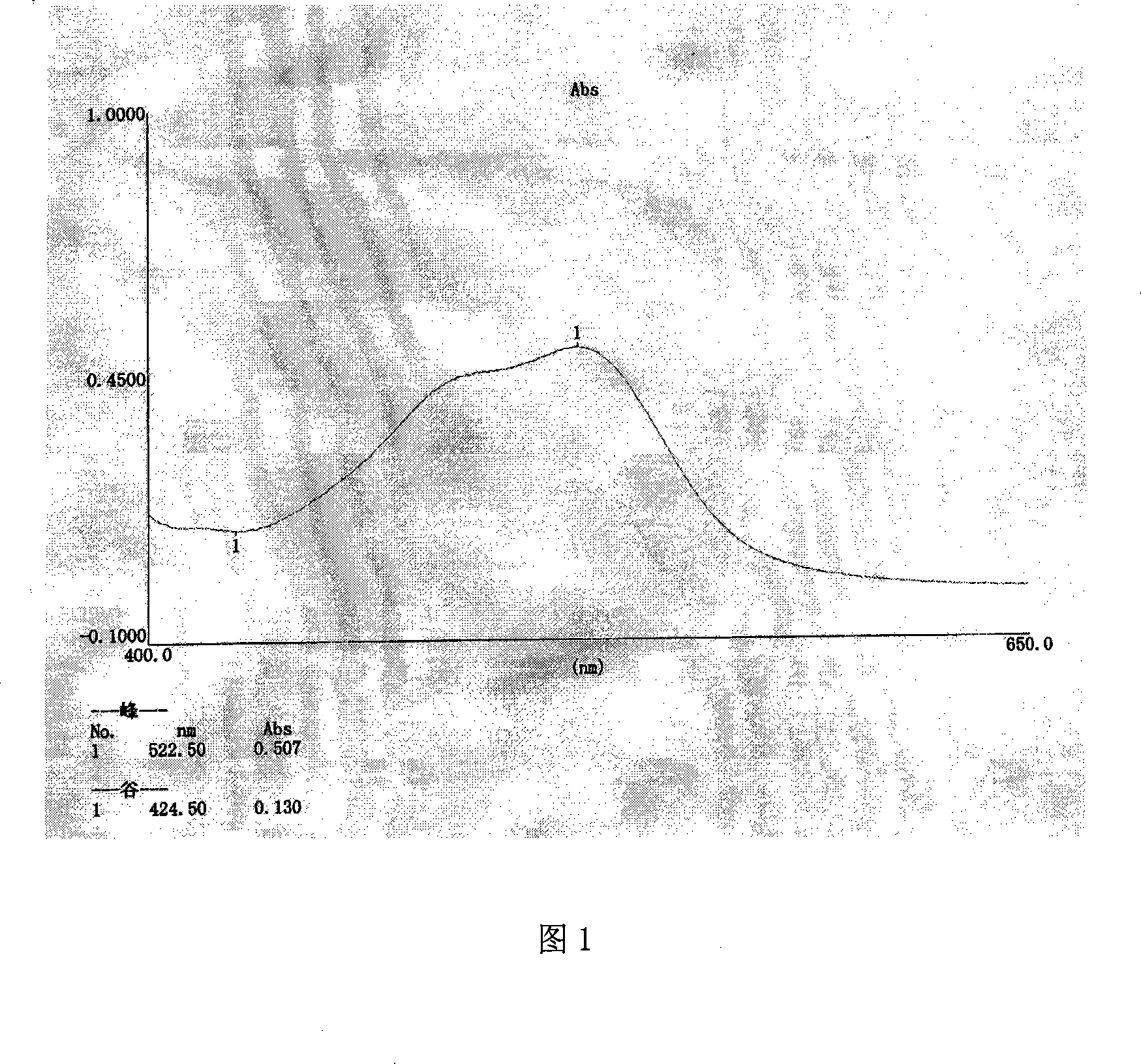
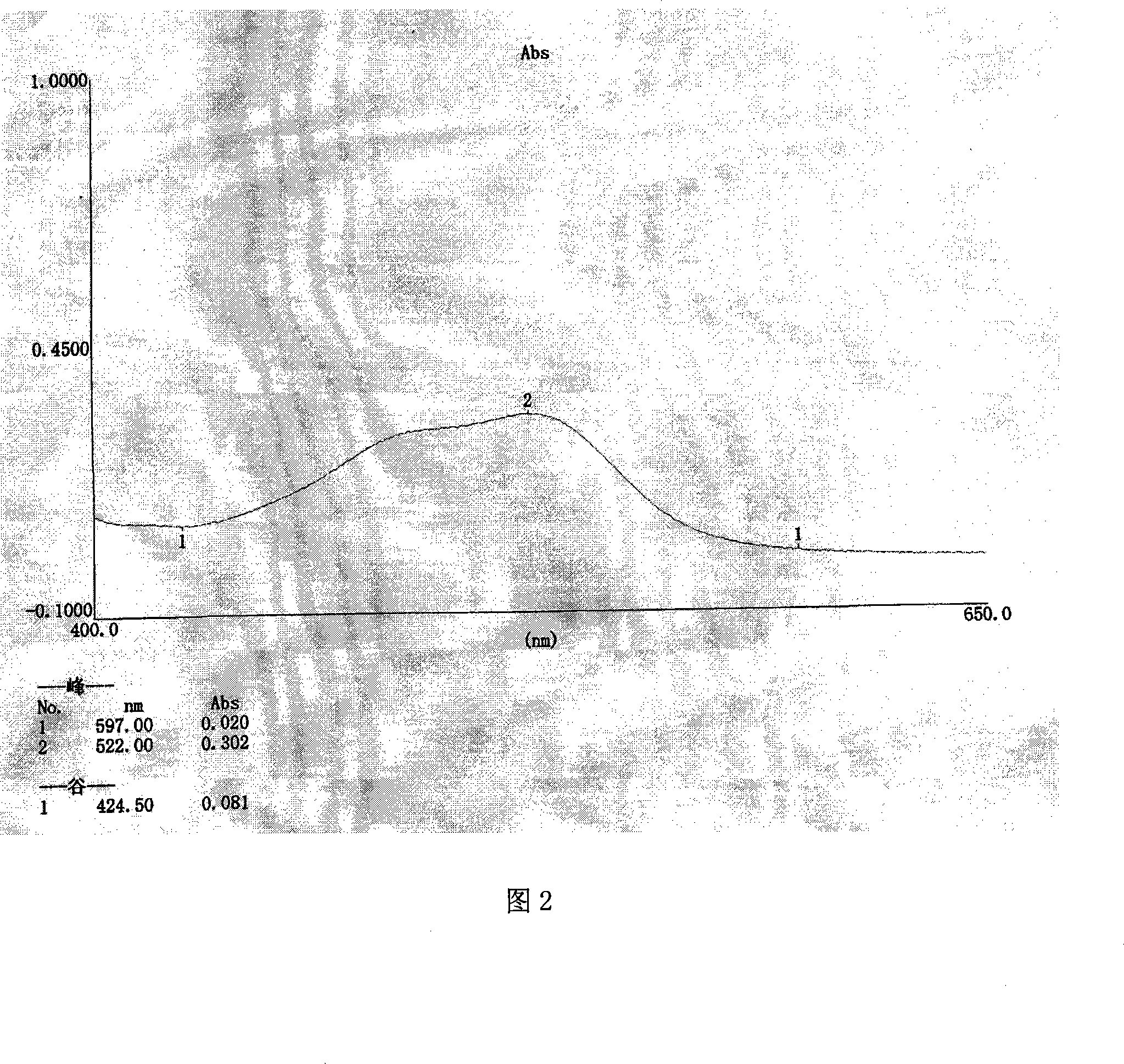
A method for detecting a generic drug pretended to be an reference listed drug comprises the following steps: step 1, obtaining reference listed drug standard spectral data and to-be-measured sample spectral data; step 2, choosing reference listed drug characteristic peaks and to-be-measured sample characteristic peaks; step three, screening matched characteristic peaks in the to-be-measured sample characteristic peaks conforming to matching conditions, and calculating the number of the matched characteristic peaks; and step four, comparing a difference value between the number of the reference listed drug characteristic peaks and the number of the matched characteristic peaks with a predetermined standard threshold t, when the difference value is less than or equal to t, judging a to-be-measured sample to be a real drug, and when the difference value is greater than t, judging the to-be-measured sample to be a fake drug, wherein t is an arbitrary integer value of 1-10. According to the method provided by the invention, the difference between the generic drug and the reference listed drug can be intuitively and comprehensively displayed, and a drug system with relatively low API content and with the other components in the drug and with relatively large interference on API characteristic peaks also can be effectively detected.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for testing quality of Nguyen supernatant pills
ActiveCN101773560AImprove quality control standardsHigh level of quality standardsInorganic boron active ingredientsHydroxy compound active ingredientsMatrineChemical reaction
The invention relates to a method for testing the quality of Nguyen supernatant pills, which are described in the 20th volume of 'Drug Standard of Ministry of Public Health of the People's Republic of China' (Chinese medicament formulation preparations). The method comprises microscopic identification method of catechu and caper, physical and chemical reactions, thin-layer chromatography identification method of matrine, thin-layer chromatography identification method of borneol, thin-layer chromatography identification method of the catechu and content measurement method of the catechu in the Nguyen supernatant pills. The method for testing the quality of the Nguyen supernatant pills improves the quality control standard of the Nguyen supernatant pills, creates the content measurement and testing index and a testing method of the catechu which is a main medicament of the preparation, deletes physical and chemical reaction identification without specificity, increases the microscopical identification method of the catechu and the capers and the thin-layer chromatography identification method of the borneol, the catechu and the matrine, and ensures high quality control level of the compound preparation.
Owner:KUNMING CHINESE MEDICINE FACTORY
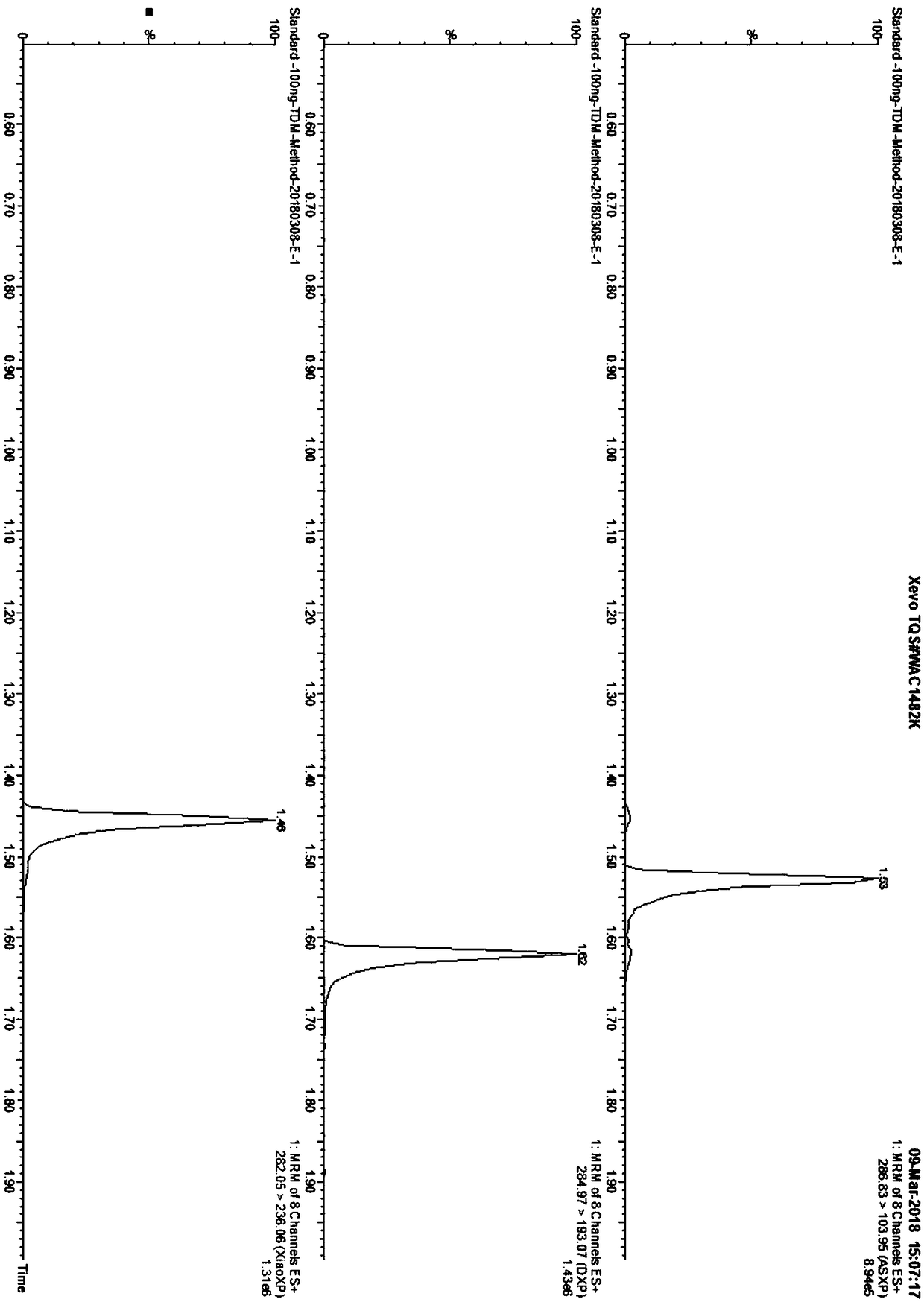
Method for detecting drugs in human bodies
The invention provides a method for detecting drugs in human bodies. The method comprises the following steps of: preparing a series of mixed drug standard solutions with gradient concentrations by using 14 drug standards; respectively performing mass spectrum detection on a sample and the mixed drug standard solutions by adopting a liquid chromatography-tandem mass spectrometry (LC-MS / MS), and determining one or more than one drugs in the mixed drug standard solutions in the sample on the basis of a mass spectrum detection result so as to achieve qualitative analysis; drawing a standard curve, detecting the peak area of a parent ion / child ion pair corresponding to at least one of the 14 drugs in the sample, and obtaining the concentration of the sample according to the standard curve for quantitative analysis, wherein the sample is optionally derived from human saliva, and optionally, the sample is pretreated. The method for detecting drugs in human bodies has the advantages of convenient material obtaining, the small dosage, the low detection limit and quantitative limit, the high accuracy, the high efficiency and the like.
Owner:BGI FORENSIC TECH (SHENZHEN) CO LTD +1
Orthosiphon n-butanol fraction medicine for treating chronic nephritis and preparation method thereof
InactiveCN102309542ATake a small doseImprove complianceUrinary disorderPlant ingredientsUse medicationPatient compliance
The invention discloses an Orthosiphon n-butanol fraction medicine for treating chronic nephritis and a preparation method thereof, belonging to the technical field of medicine. The content of phenolic acid in the Orthosiphon n-butanol fraction is 50-60 wt%. The preparation method comprises the following steps: boiling Orthosiphon extraction liquid and carrying out condensation, extracting with chloroform, and then extracting with n-butanol. According to the invention, the active site of treating chronic nephritis by Orthosiphon is the n-butanol fraction, the composition and content of the ingredients are clear; by extraction and purification, ineffective substances for treating chronic nephritis are removed, the dosage is reduced, and patients compliance with medication is increased. Simultaneously, the invention is good for the establishment of drug standard and quality control, and is convenient for development and application of various preparations.
Owner:SHANGHAI JIAO TONG UNIV
Method for optimizing freeze-drying curve of urokinase freeze-drying preparation for injection
InactiveCN109959227AMaintain biological activityOptimizing the freeze-drying profileDrying solid materials without heatFreeze-dryingMedicine
The invention discloses a method for optimizing a freeze-drying curve of a urokinase freeze-drying preparation for injection. According to the technical scheme, reasonable pre-freezing and sublimationtemperatures and reasonable vacuum degrees during drying are selected to improve the properties of freeze-dried products. The quality standards of the freeze-dried products conform to various indexesspecified by the national drug standard. The method has the advantages of stable characters, attractive appearance and the like for freeze-dried products.
Owner:青岛华迈士药业有限公司
Method for detecting quality of lung clearing and phlegm eliminating pill
ActiveCN101732552AImprove quality control standardsHigh level of quality standardsComponent separationPill deliveryPublic healthMedicine
The invention relates to a method for detecting quality of a lung clearing and phlegm eliminating pill in Volume 12 of Drug Standard of Ministry of Public Health of Peoples Republic of China (traditional medicine prescribed preparation). The method for detecting quality of a lung clearing and phlegm eliminating pill comprises microscopic identification, a thin layer chromatography identification method for orange peel and bitter orange, a thin layer chromatography identification method for ephedrine hydrochloride in herba ephedrae, a thin layer chromatography qualitative identification method for balloon flower, and scutelloside content measurement. The invention enhances the quality control standard of the lung clearing and phlegm eliminating pill, establishes content measuring and detecting indexes in main medicines of preparation and a detecting method thereof, increases the thin layer chromatography qualitative identification methods for orange peel, bitter orange, ephedrine hydrochloride and balloon flower, and ensures the higher quality standard levels of the compound preparation.
Owner:KUNMING CHINESE MEDICINE FACTORY
Detection method of traditional Chinese medicine preparations
ActiveCN105628851ASimplify usageEasy to operateComponent separationPreparing sample for investigationSalvianolic acid BCurative effect
The invention discloses a detection method of traditional Chinese medicine preparations. The detection method is used for detecting active ingredient content of f traditional Chinese medicine preparations, ensuring that medicine quality accords with drug standards, and ensuring stability of curative effect of the traditional Chinese medicine preparations. The detection method comprises following steps: TLC is adopted for qualitative identification of radix achyranthis bidentatae, radix salviae miltiorrhizae, fructus gardenia, herba epimedii, and semen cassia in the traditional Chinese medicine preparations, and HPLC is adopted for detection of contents of salvianolic acid B and gastrodin in the traditional Chinese medicine preparations.
Owner:JIUZHITANG
Novel medical hot melt resin carrier gel matrix and its preparation method and application
ActiveCN101288772AStimulating smallNo allergiesOil/fats/waxes non-active ingredientsSheet deliveryDrug releaseSkin irritant
The invention provides a novel medicinal thermal melting resin vector adhesive matrix which is made from a thermoplastic elastomer, medical resin, softerner, skin transdermal enhancer and excipient which are mixed in a way of melting according to a certain percentage. The novel medicinal thermal melting resin adhesive prepared by the invention is a substitute for traditional black plaster, adhesive plasters, cataplasm and attached agent matrix excipient. The novel medicinal thermal melting resin vector adhesive matrix of the invention achieves the requirement of new medicinal excipient declared by SFDA in aspects of preparation process, production equipment, drug standard, pilot-scale study, drug stability, experimental storage, etc. Emplastrum as the matrix which is produced by the novel medicinal thermal melting resin vector adhesive of the invention has the characteristics of large drug loading, fast drug releasing rate, high stability, small skin irritation, short production cycle, high efficiency, safety, environmental protection, etc.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Freeze-dried powder injection of vitamin B6 and preparation method thereof
ActiveCN102871971AAvoid content (potency) reduction problemsReduce contentPowder deliveryOrganic active ingredientsArginineFreeze-drying
The invention relates to a freeze-dried powder injection of vitamin B6 and a preparation method thereof. The freeze-dried powder injection is made from a 1000 ml water solution comprising the following components in parts by weight: 50-200 parts of the vitamin B6, 5-10 parts of L-arginine, 10-20 parts of mannitoland 2-6 parts of phosphoric acid buffer salt. The finally prepared freeze-dried powder injection of the vitamin B6 meets requirements of national drug standards by adopting the composition in the preparation method, namely the proportion.
Owner:MAANSHAN BBCA PHARMA
Traditional Chinese medicine granule for treating postpartum lochiorrhea and lesser-abdominal pain and preparation method thereof
InactiveCN105362975AImprove stabilityIncrease profitAntipyreticAnalgesicsMedicinal herbsAngelica Sinensis Root
The invention provides a traditional Chinese medicine granule for treating postpartum lochiorrhea and lesser-abdominal pain and a preparation method thereof, and belongs to the field of medicine. The granule and the preparation method overcome the defects that according to an existing preparation method, the volatile oil extraction rate is low, the utilization rate is low, losses of effective components of medicine materials are large, particle appearance is not good, and dissolubility is poor. The preparation method includes the steps that seven decoction pieces of angelica sinensis, rhizome chuanxiong, peach kernels, honey-fried licorice roots, ginger charcoal, dried leonurus and safflower are obtained according to the formula of new granule for postpartm troubles (the fifth volume of drug standard traditional Chinese medicine prescription preparation of Ministry of Health of the People's Republic of China), the materials are smashed into coarse powder to extract volatile oil through a microwave method, a mixed volatile oil cyclodextrin clathrate compound is prepared through a colloid milling method, residues are decocted twice with water, decocted liquid of the first time and decocted liquid of the second time are combined and filtered, filter liquid is refrigerated, supernate is sucked to be subjected to centrifugation twice through a tubular centrifugal machine, then supernate is decompressed and concentrated to be clear ointment with the relative density being 1.05-1.30 (60 DEG C), the clear ointment is added into the cyclodextrin clathrate compound and evenly mixed into suspension, the suspension is sprayed, granulated, screened and subpackaged, and the granule is obtained. The invention further discloses application of novel particles for postpartm troubles in the aspect of treatment of climacteric syndromes.
Owner:JIANGSU TIANZHAO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com