Quinolone drug chemiluminescence enzyme-linked immunodetection kit
A technology of chemiluminescent enzymes and quinolones, which is applied in the field of immunological detection, can solve problems such as inability to achieve, and achieve the effect of increased sensitivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of derivatives, immunogens, coating agents and monoclonal antibodies
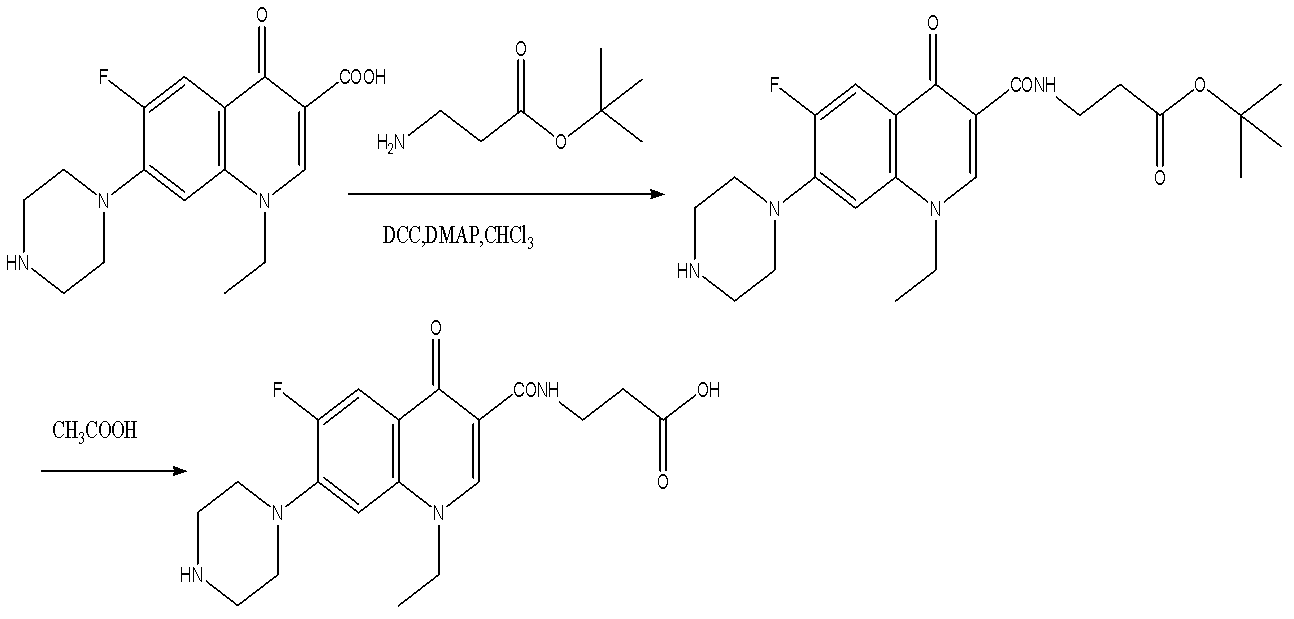
[0047] (1) Synthesis of norfloxacin derivatives
[0048] A. Dissolve 1 mmol of norfloxacin in 15 mL of chloroform, add 2 mmol of DCC, catalytic amount of DMAP, 1.5 mmol of tert-butyl allanine, stir at room temperature for 5 h, monitor the disappearance of raw materials by TLC, filter, wash the liquid phase with water, Dry with water NaS2O4, and purify by column chromatography (eluent, ethyl acetate / petroleum ether, 1 / 5).
[0049] B. Dissolve the above product in glacial acetic acid, stir at room temperature for 2 hours, monitor the disappearance of raw materials by TLC, remove the solvent under reduced pressure, dissolve the obtained viscous product in 1mol / L NaOH solution, adjust the pH to 3~5, extract with ethyl acetate, and dry. Purify by column chromatography (eluent, ethyl acetate / petroleum ether, 1 / 1) to obtain quinolone haptens.
[0050] (2) Synthesis of immunogen
[...
Embodiment 2
[0060] Embodiment 2: the establishment of ELISA detection method
[0061] (1) Optimization of antibody and coated antigen concentration (square matrix)
[0062] Serially dilute each coated antigen longitudinally at 80.0 μg / mL, 40.0 μg / mL, 20.0 μg / mL, 10.0 μg / mL, 5.0 μg / mL, 2.5 μg / mL, 1.25 μg / mL, 0.625 μg / mL Coat the ELISA plate with 100 μL / well, place it in a 37°C incubator for 2 hours, and then pat it dry; seal it with 150 μL / well blocking solution, place it in a 37°C incubator for 2 hours, wash the plate once, and pat it dry; add 50 μL / well A series of diluted antibodies (1:1000 to 1:512000), then add 50 μL / well of 1:2000 horseradish peroxidase-goat anti-mouse IgG antibody, incubate at room temperature (20-25°C) for 15 minutes, wash the plate 5 pat dry for the last time; add 100 μL / well of chemiluminescence solution, and measure the luminescence intensity value. Specificity determination was carried out with the coating antigen concentration and antibody dilution having ob...
Embodiment 3
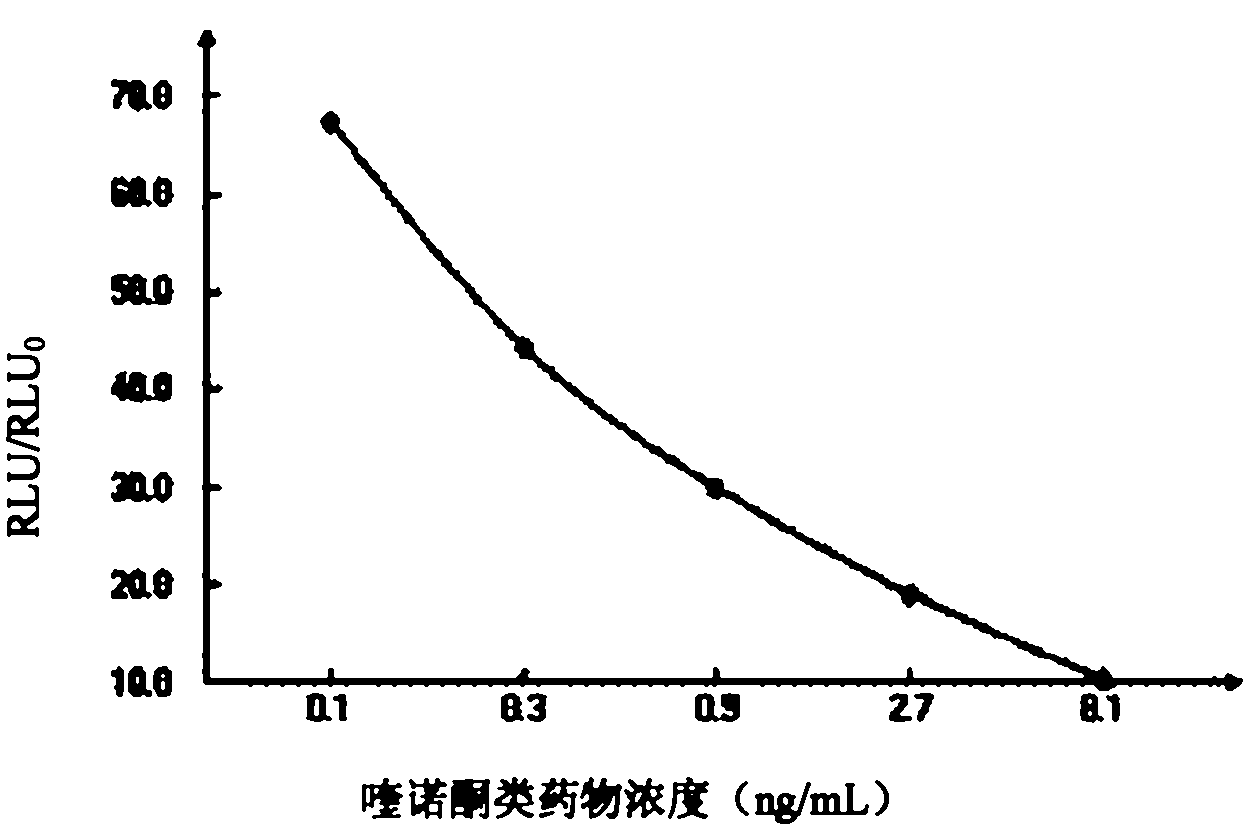
[0072] Example 3: Chemiluminescence enzyme-linked immunosorbent assay kit for detecting quinolones
[0073] (1) The composition of the chemiluminescent ELISA kit for detecting quinolones
[0074] A, the solid phase carrier (elisa plate) that is coated with coating former (QNS-OVA);
[0075] B, Standard solutions of quinolones: 0ng / mL, 0.1ng / mL, 0.3ng / mL, 0.9ng / mL, 2.7ng / mL and 8.1ng / mL.
[0076] C. Enzyme-labeled goat anti-mouse antibody solution: Enzyme-labeled goat anti-mouse antibody is horseradish peroxidase-goat anti-mouse IgG stock solution, filled in, and prepared with washing solution to a working concentration of 1:2000 when used.
[0077] D, quinolone drug antibody solution: the monoclonal antibody prepared by immunizing animals with artificial immune antigen (QNS-BSA), and the obtained quinolone drug antibody was diluted with washing solution to a working concentration of 1:32000.
[0078] E, luminescent solution: A solution is tris(hydroxymethyl)aminomethane solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com