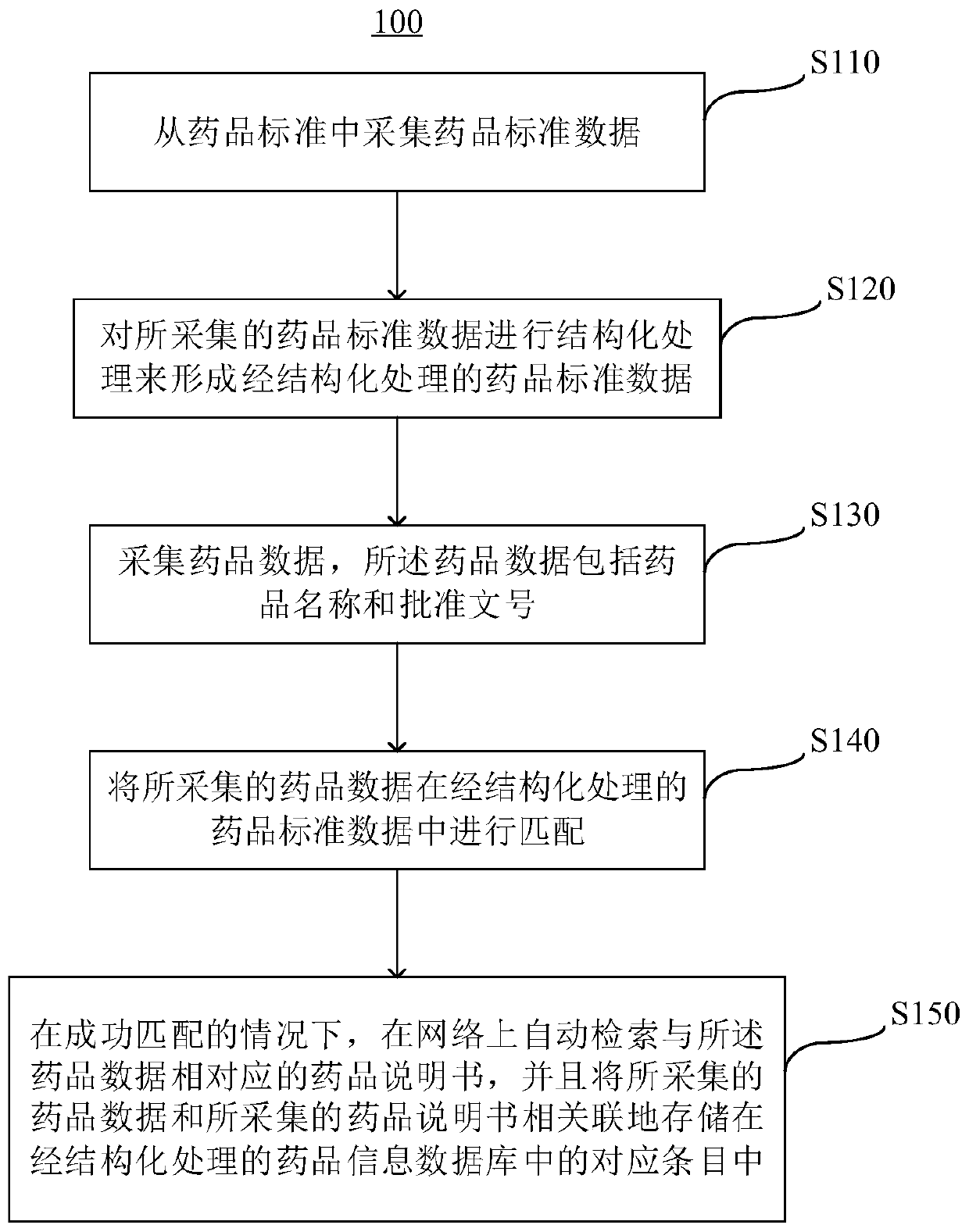
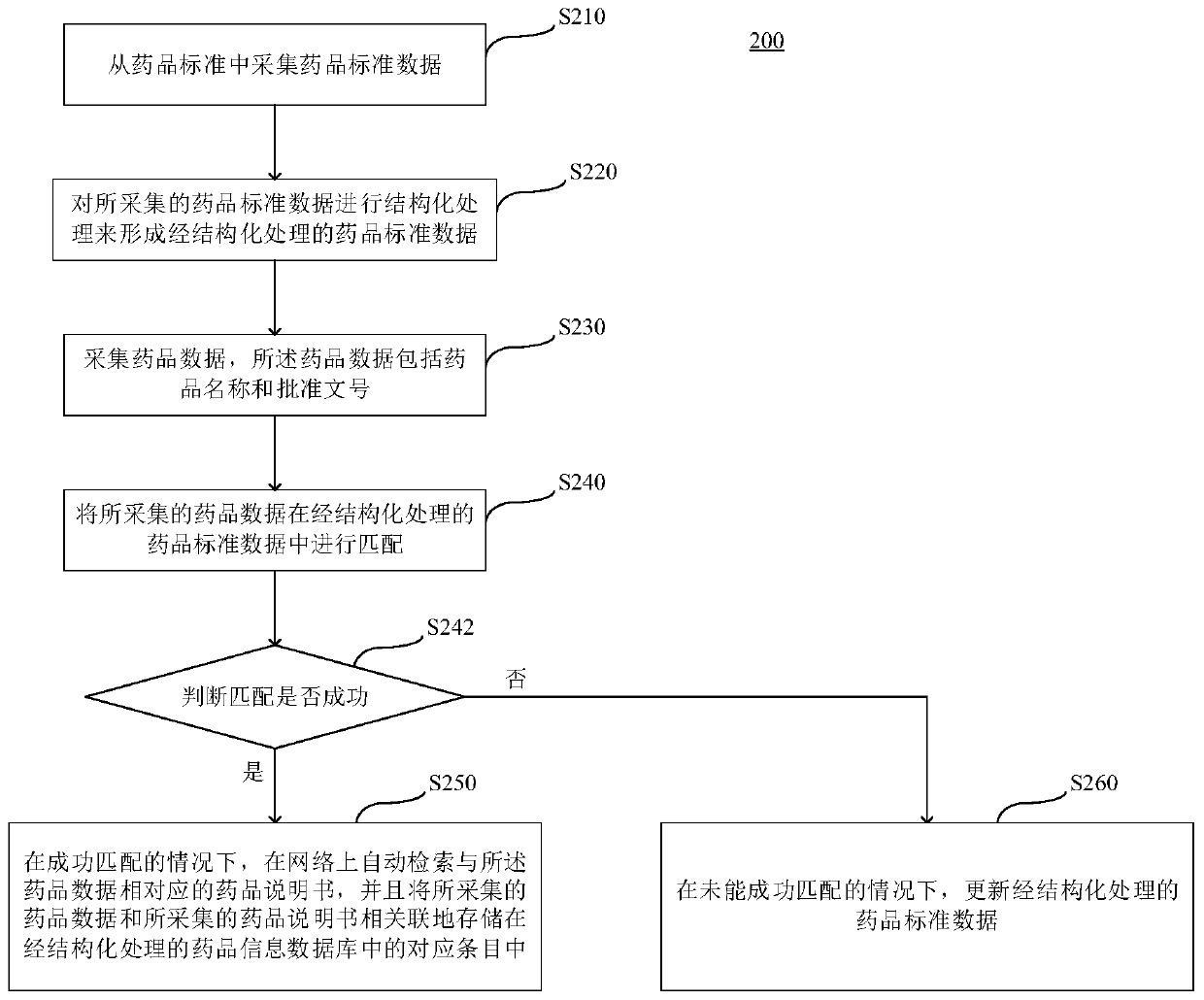
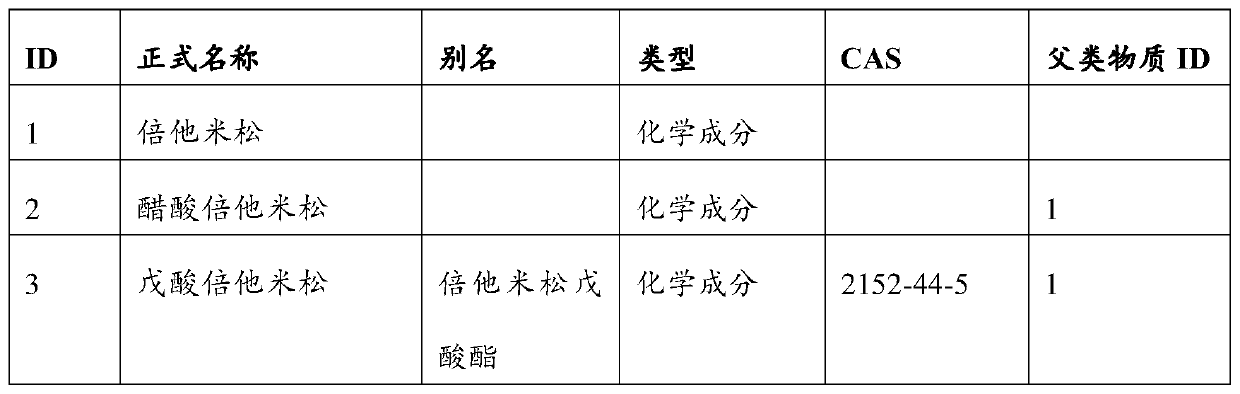
Marketed drug information standardization method and device, server and storage medium
A drug standard and drug information technology, applied in the direction of instruments, drug reference, electronic digital data processing, etc., can solve the problems of unguaranteed data quality, incomplete drug information, and inability to ensure high coverage of drug information, etc., to achieve fast and comprehensive Collect and deliver effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] In the following, only some exemplary embodiments are briefly described. As those skilled in the art would realize, the described embodiments may be modified in various different ways, all without departing from the spirit or scope of the present disclosure. Accordingly, the drawings and descriptions are to be regarded as illustrative in nature and not restrictive.
[0040] The following disclosure provides many different embodiments or examples for implementing different structures of the present disclosure. To simplify the disclosure of the present disclosure, components and arrangements of specific examples are described below. Of course, they are merely examples, and are not intended to limit the present disclosure. Furthermore, the present disclosure may repeat reference numerals and / or reference letters in different instances, such repetition is for simplicity and clarity and does not in itself indicate a relationship between the various embodiments and / or arran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com