Quality standard and test method of hoove pill and preparation threrewith
A quality control method and preparation technology, which are applied in the directions of pill delivery, measuring device, pharmaceutical formula, etc., can solve the problems of no content determination index, few detection indicators, and inability to effectively control product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
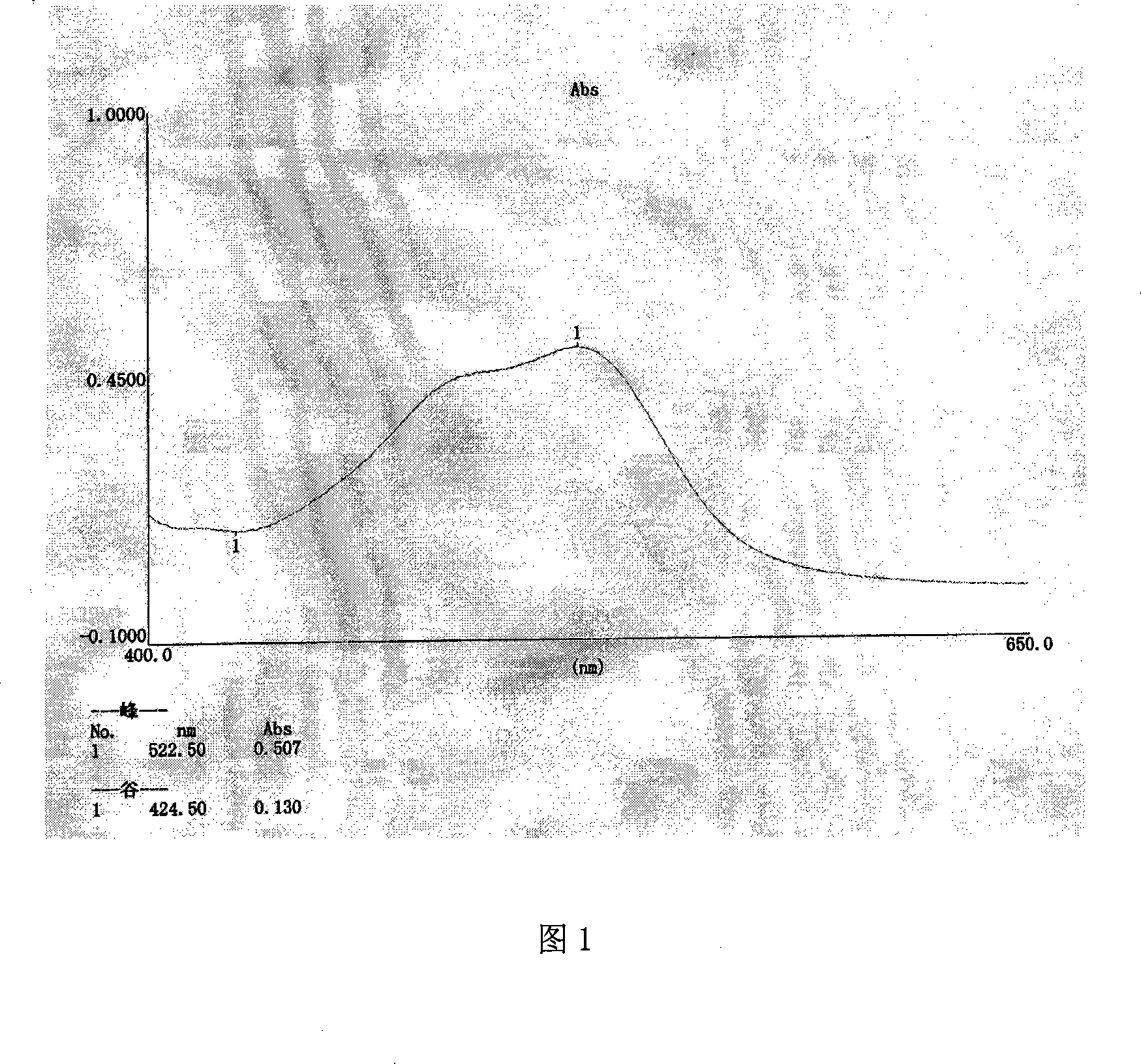
[0077] Embodiment 1: the identification method and content determination method of pharmaceutical composition pill of the present invention
[0078] Identification: A. Get 8g of the pharmaceutical composition pill of the present invention, grind it finely, add methanol 70ml, ultrasonically treat it for 20 minutes, filter, evaporate the filtrate to dryness, add 35ml of water to the residue to dissolve, extract three times with n-butanol, each solvent is 15ml , combine the extracts, evaporate to dryness, add 1ml of methanol to the residue to dissolve, and use it as the test solution; take 7g of Kansui reference medicinal material, add 35ml of methanol, ultrasonicate for 50 minutes, filter, evaporate the filtrate to dryness, add 1ml of methanol to the residue to make Dissolve, as contrast medicinal material solution; According to Chinese Pharmacopoeia 2005 edition an appendix VI B thin-layer chromatography test, draw need testing solution 0.006ml, contrast medicinal material solut...
Embodiment 2
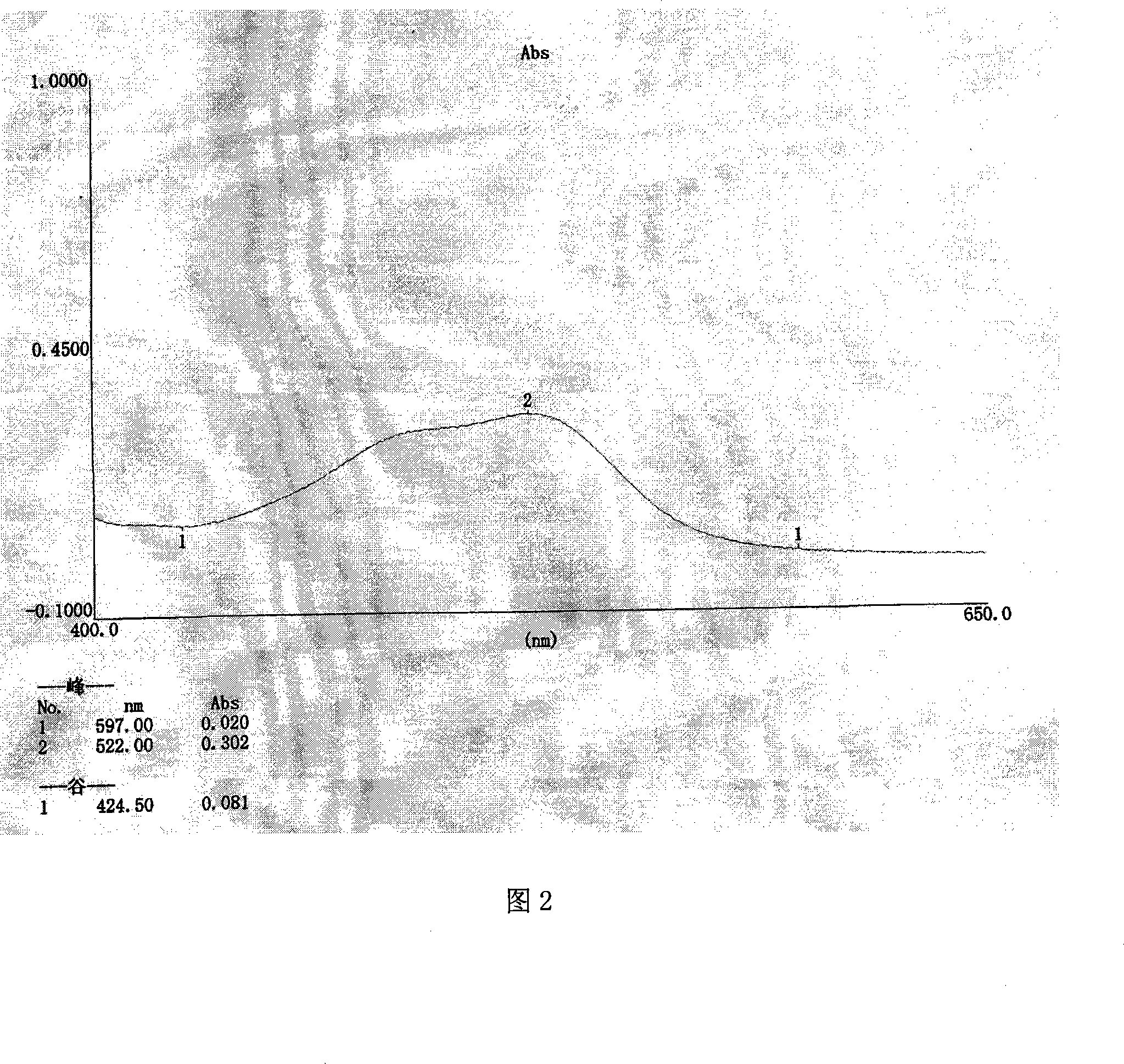
[0085] Embodiment 2: the identification method and assay method of pharmaceutical composition tablet of the present invention
[0086]A. Get 20g of the tablet of the pharmaceutical composition of the present invention, grind it finely, add 60ml of ethyl acetate, heat reflux extraction for 40 minutes, filter, evaporate the filtrate to dryness, add 30ml of water to the residue to dissolve, extract three times with ethyl acetate, each solvent to 20ml, combine the extracts, evaporate to dryness, add 1ml of ethanol to the residue to dissolve, and use it as the test solution; take 3g of Gansui reference medicinal material, add 40ml of ethyl acetate, extract under reflux for 40 minutes, filter, and evaporate the filtrate to dryness. Add 1ml of ethyl acetate to the residue to dissolve it, and use it as a reference medicinal solution; according to the Chinese Pharmacopoeia 2005 Edition, Appendix VI B thin-layer chromatography test, absorb 0.01ml of the test solution and 0.005ml of the r...
Embodiment 3
[0092] Embodiment 3: the identification method of pharmaceutical composition oral liquid preparation of the present invention:
[0093] Take 25ml of the oral liquid preparation of the pharmaceutical composition of the present invention, add 50ml of petroleum ether with a boiling range of 60 to 90°C, extract by cold soaking for 40 minutes, filter, evaporate the filtrate to dryness, add 1ml of n-butanol to the residue to dissolve, and use it as the test solution Take another 3 g of the reference medicinal material of Muxiang, add 10 ml of methanol, soak in cold for 40 minutes, filter, evaporate the filtrate to dryness, add 1 ml of ethanol to the medicinal residue to dissolve, and use it as the reference medicinal material solution; Chromatography test, draw 0.01ml of each of the above two solutions, respectively spot on the same silica gel G thin-layer plate, use cyclohexane: acetone = 9:2 solution as developing agent, develop, take out, dry in the air, spray with 10 % sulfuric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com