Patents
Literature
38results about How to "Bioadhesive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
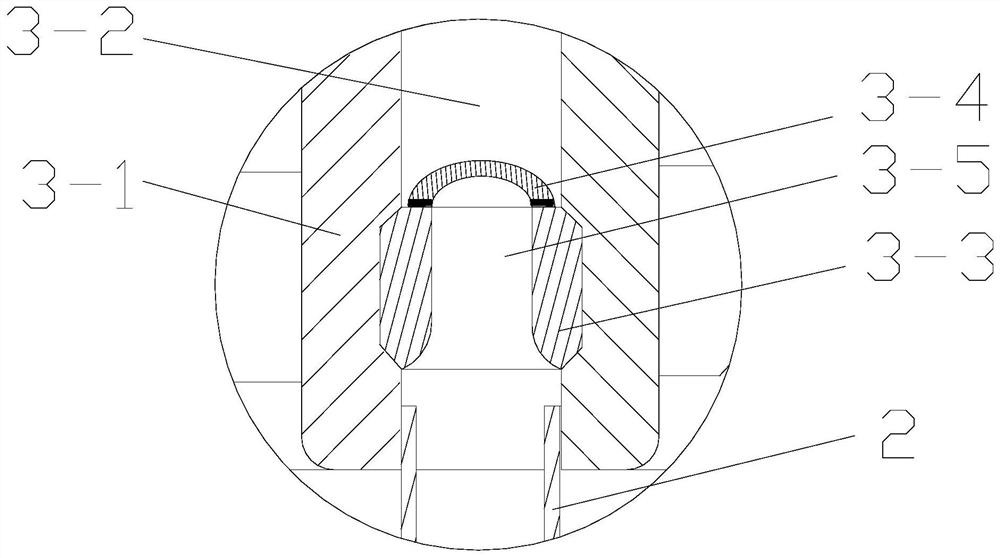
Ultra porous hydrogel complex substance, preparing method and use in pharmaceutics thereof
InactiveCN1488331AHigh mechanical strengthGood bioadhesionPeptide/protein ingredientsPharmaceutical delivery mechanismCross-linkFoaming agent
The invention discloses an ultra-multiaperture hydrogel compound, making method and application in pharmacy. It contains cross-linked polymer and Carbomer. At least one unsaturated alkene monomer and polyene cross-linking agent polymerize to develop it. The making steps: mix at least one unsaturated alkene monomer, one polyene cross-linking agent, Carbomer and one foaming agent; the mixture develops it on the condition of polymerizing and foaming. It can be used in stomach floating preparation and protein polypeptide oral medicine supplying system.
Owner:FUDAN UNIV
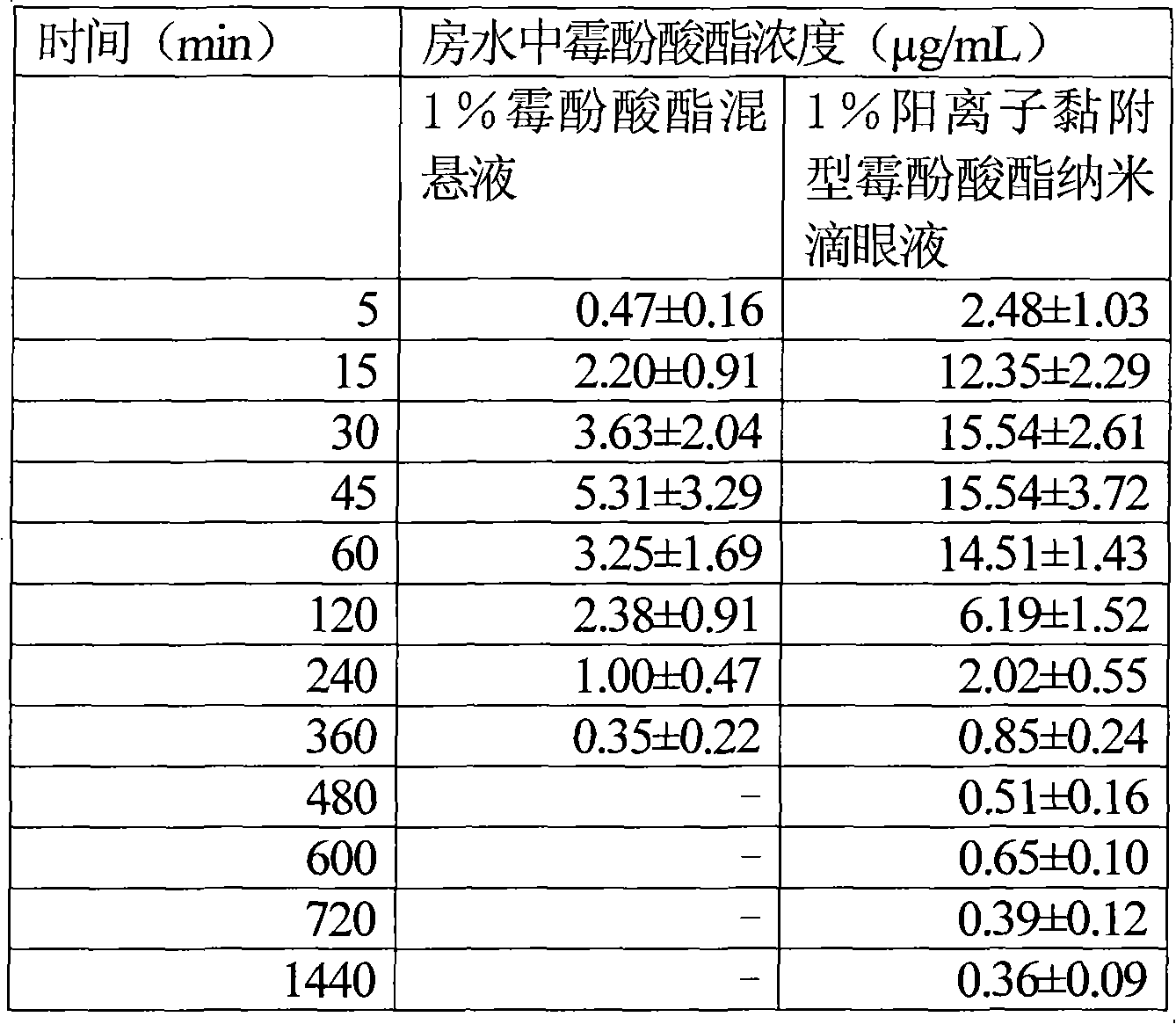
Cation adhesion type natamycin nano eye drops
ActiveCN101385703AImprove solubilityIncrease corneal resorptionOrganic active ingredientsSenses disorderSolubilityPhospholipid
A cationic adhesive-type natamycin nanometer eye drop comprises natamycin, a proper amount of osmoregulator and pH regulator. The eye drop is characterized by further comprising chitosan, pluronic F-68 and phospholipid, wherein, the mass percent concentration of the phospholipid in the eye drop is 0.01-2%, the mass percent concentration of the pluronic F-68 in the eye drop is 0.1-20%, and the mass percent concentration of the chitosan in the eye drop is 0.01-4%. The molecular weight of the chitosan is 5000-300000, and the deacetylation degree of the chitosan is greater than 85%. The natamycin nanometer eye drop is characterized by making a common natamycin suspension into suspension particles with the diameter ranging from 10nm to 1000nm, thus increasing solubility of the eye drop and enhancing safety and effectiveness. The natamycin particles of the common suspension are modified by the phospholipid and the chitosan to cause the particles to be positively charged, which is helpful to improve absorption of the eye drop by cornea, enhances drug effect and prolongs the action time of the eye drop. And the chitosan has bioadhesion function, which enhances the adhesiveness between medicinal particles and the cornea.
Owner:SHANDONG EYE INST
Phosphorus-based biochar material, and preparation and application thereof
ActiveCN105598158ALarge specific surface areaBioadhesiveContaminated soil reclamationMaterials preparationCarbonization
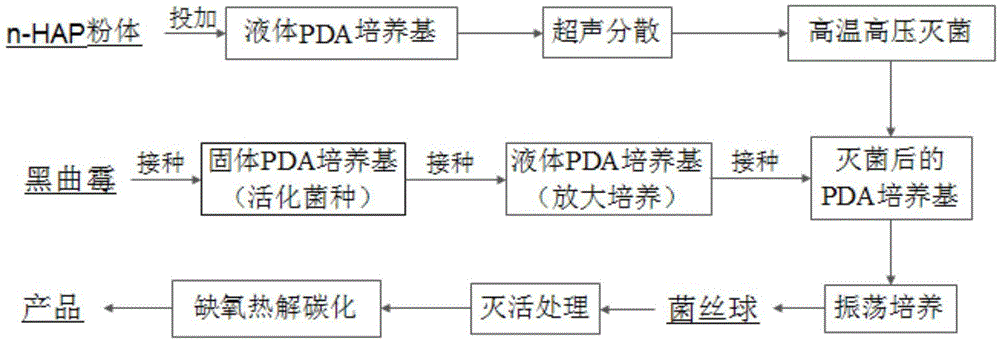
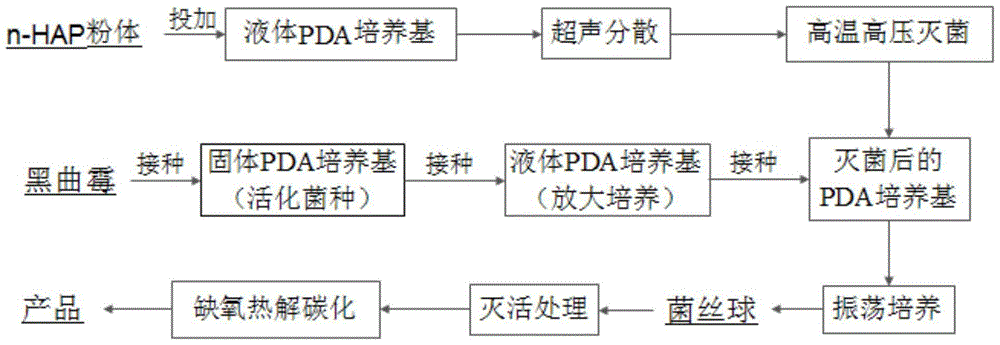
The invention discloses a phosphorus-based biochar material, and preparation and application thereof. The phosphorus-based biochar material with a special structure and a special function is formed by performing anoxia high-temperature carbonization on mycelium pellets which are wrapped with nano hydroxyapatite and serve as a raw material. The synthesized phosphorus-based biochar material is uniformly mixed with soil according to a certain ratio to obtain a mixture; and after the mixture is cured for 7 days, the content of cadmium in an effective state in the soil is reduced by 44.8 to 50.1 percent, so that the treatment effect of the phosphorus-based biochar material is better than that of a pure-phosphorus-based material. The material preparation process is simple, and the cost is lower; biological degradation can be realized, and no secondary pollution is caused; and therefore, the phosphorus-based biochar material is an environment-friendly material.
Owner:CENT SOUTH UNIV
Application of phosphorus base charcoal material to remediation of lead contaminated soil
ActiveCN105689374ALarge specific surface areaBioadhesiveContaminated soil reclamationCarbonizationPollution
The invention discloses application of a phosphorus base charcoal material to remediation of lead contaminated soil. According to the phosphorus base charcoal material, microorganisms are used as raw materials; and a phosphorus base material with a specific ratio is doped to form the phosphorus base charcoal material with special structure and functions through anaerobic high-temperature carbonization. The material is simple in preparation process, lower in cost, biodegradable and free of secondary pollution; and the obtained phosphorus base charcoal material has a better effect on remediation of the lead contaminated soil, and the bioavailability of lead in the soil can be effectively reduced. The synthesized phosphorus base charcoal material is uniformly mixed with soil by a certain ratio; and after fixation by 7 days, available lead in the soil is reduced by 33.0-59.8%, so that the phosphorus base charcoal material has a better remediation effect compared with a pure phosphorus base material.
Owner:CENT SOUTH UNIV
Composition for preventing or treating mucous membrane diseases and preparation method thereof
InactiveCN106310266ABioadhesiveEfficient killingAntibacterial agentsAntimycoticsEscherichia coliNasal cavity
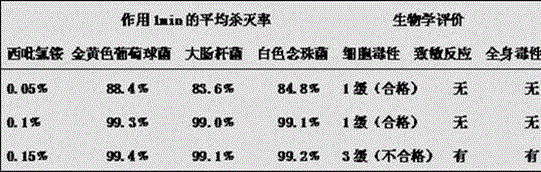
The invention discloses a composition for preventing and treating mucous membrane diseases with staphylococcus aureus, escherichia coli and candida albicans as pathogenic bacteria and a preparation method thereof. The composition is characterized by being prepared from the following components in percentage by mass: 0.001-35 percent of an antimicrobial agent, 0-10.0 percent of an analgesic, 0.001-20.0 percent of a bio-adhesive, 0-30.0 percent of a humectant, 0-5.0 percent of a pH regulator, 0-8.0 percent of a flavoring agent, 0-5.0 percent of an emulsifier, 0-8.0 percent of an auxiliary antimicrobial agent and the balance of pure water, totally 100 percent. The components in the composition can be well combined to achieve functions of inhibiting bacteria, isolating, relieving pain and moistening, and the effects of isolating microbial infection, preventing secondary infection and accelerating wound healing can be achieved. The composition is especially suitable for preventing and treating mucous membrane diseases in the oral cavity, nasal cavity, gastrointestinal tracts, respiratory tracts, vagina, urinary bladder, genitals, epidermis and other positions.
Owner:王强 +1
Iron-based biochar material as well as preparation and application thereof
ActiveCN105733588AHigh adsorption capacityReduce manufacturing costContaminated soil reclamationOrganic fertilisersToxicityWater soluble
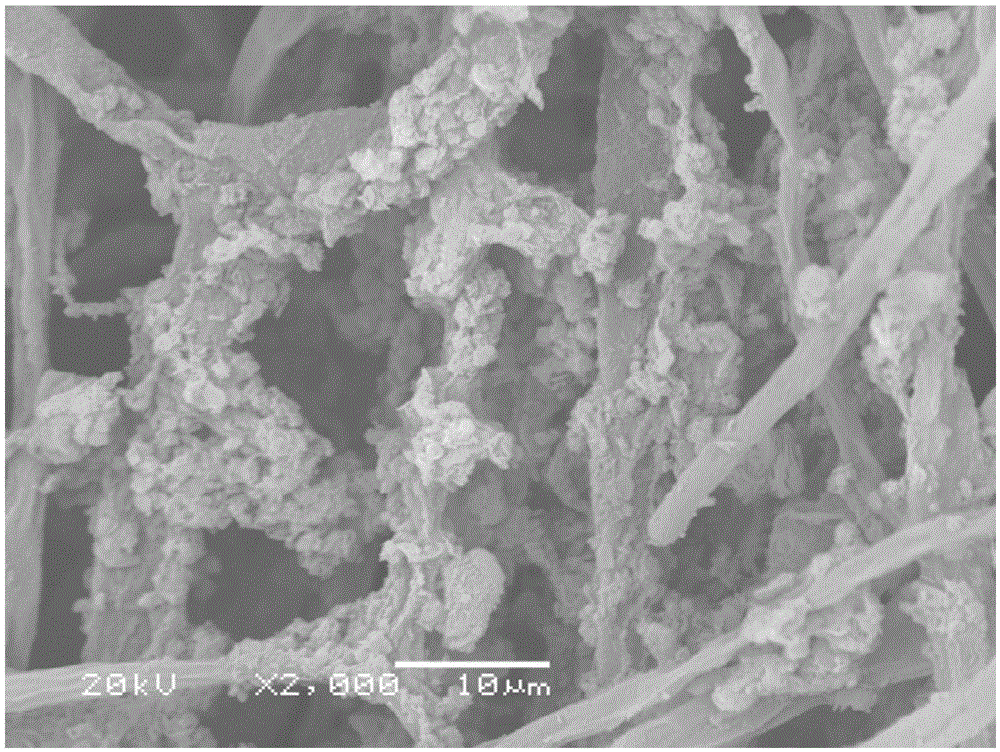
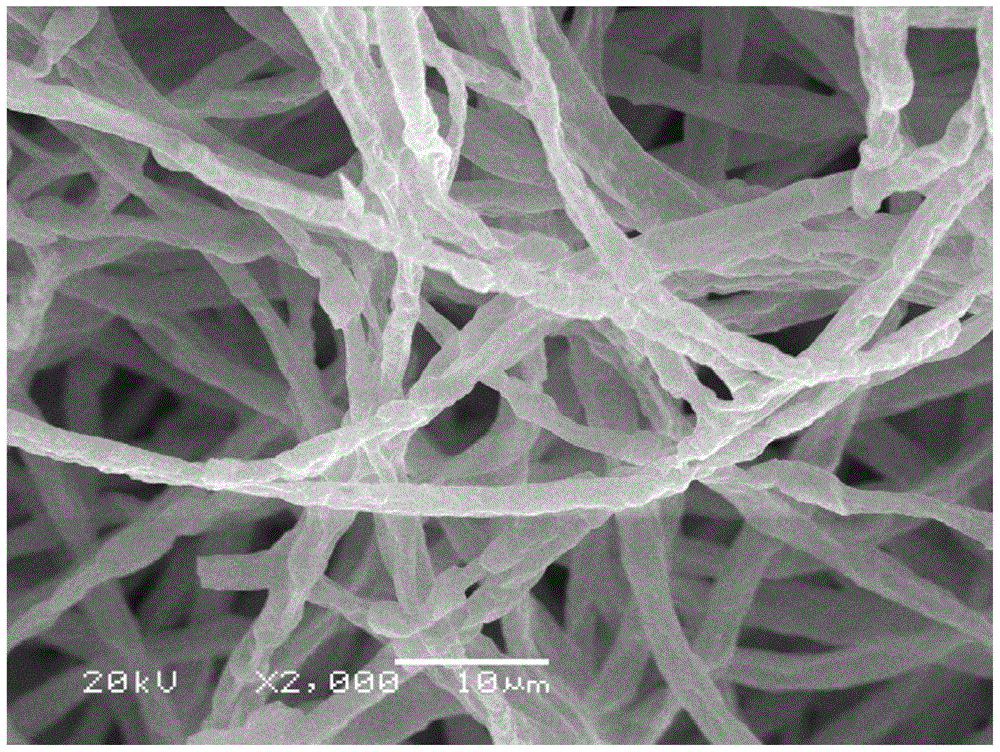
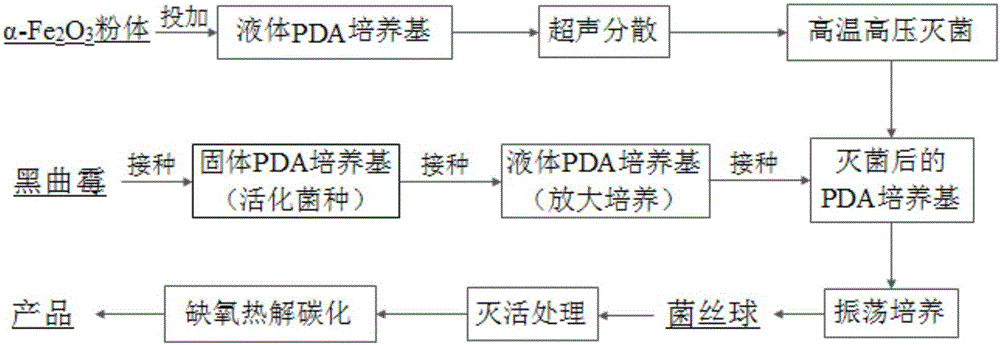
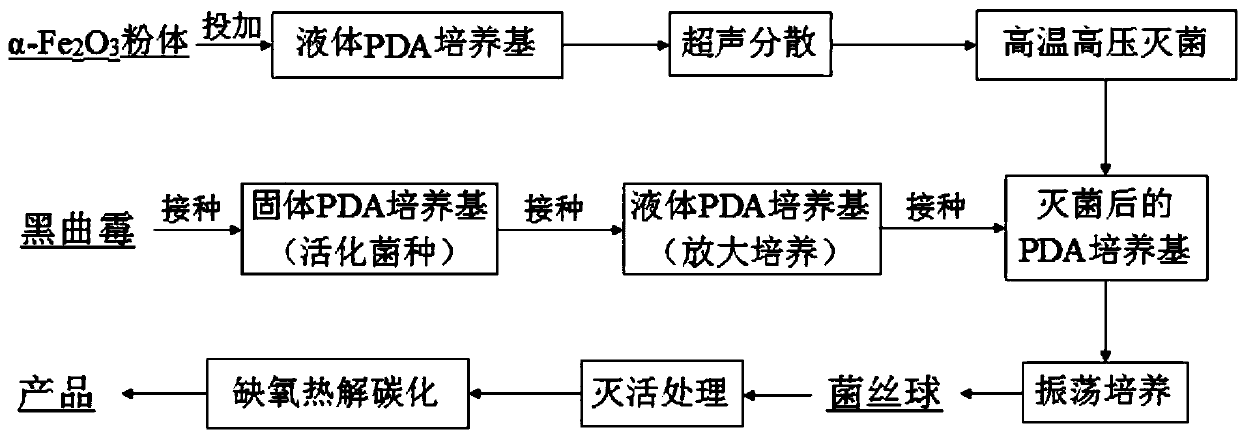
The invention discloses an iron-based biochar material as well as preparation and application thereof. In the invention, by adopting nano alpha-Fe2O3 as a center substance and wrapping the nano alpha-Fe2O3 with aspergillus niger hyphe, the iron-based biochar material for arsenic-polluted soil remediation is prepared through anoxic high-temperature carbonization. The synthesized iron-based biochar material is uniformly mixed with soil according to certain proportion; and after a period of time, the fixed rate of the water-soluble state arsenic and effective-state arsenic in the arsenic-polluted soil reaches 59.6% and 66.2% respectively. The iron-based biochar material disclosed by the invention has the advantages of easiness in preparation, high efficiency without toxicity, biodegradability and no secondary pollution or damage of physicochemical properties of soil and is an environment-friendly fixing agent.
Owner:CENT SOUTH UNIV
Anti-fungus and mothproof coating for textiles
The invention discloses an anti-fungus and mothproof coating for textiles, comprising the following components by weight parts: 10-30 parts of polyurethane, 10-20 parts of pyridine copper sulfate, 10-15 parts of alcohol, 1-10 parts of chitosan and 1-5 parts of sodium tripolyphosphate. The coating is prepared by the following steps of: dissolving the polyurethane in the alcohol, dissolving the chitosan in 0.5-1.5 wt% of acetum, dissolving the pyridine copper sulfate in chitosan solution, then, adding the polyurethane alcohol solution into the chitosan solution, slowing adding the sodium tripolyphosphate, simultaneously, stirring at high speed and executing ultrasonic processing, finally, obtaining the coating. The anti-fungus and mothproof coating has the following positive effects that: the coating is high-efficiency, low-toxicity, broad-spectrum, long-acting, antibacterial to various microorganisms, strong in antibiotic property and high in safety, can keep integrity and efficiency for longer time, and has no influence on strength of gray fabrics.
Owner:WUJIANG RUIFENG WEAVING
Amphiphilic chitosan derivative drug-loaded nano-micelle and preparation method
InactiveCN105687133AProlong blood circulation timeGood biocompatibilityOrganic active ingredientsAntiviralsChemistrySolubility
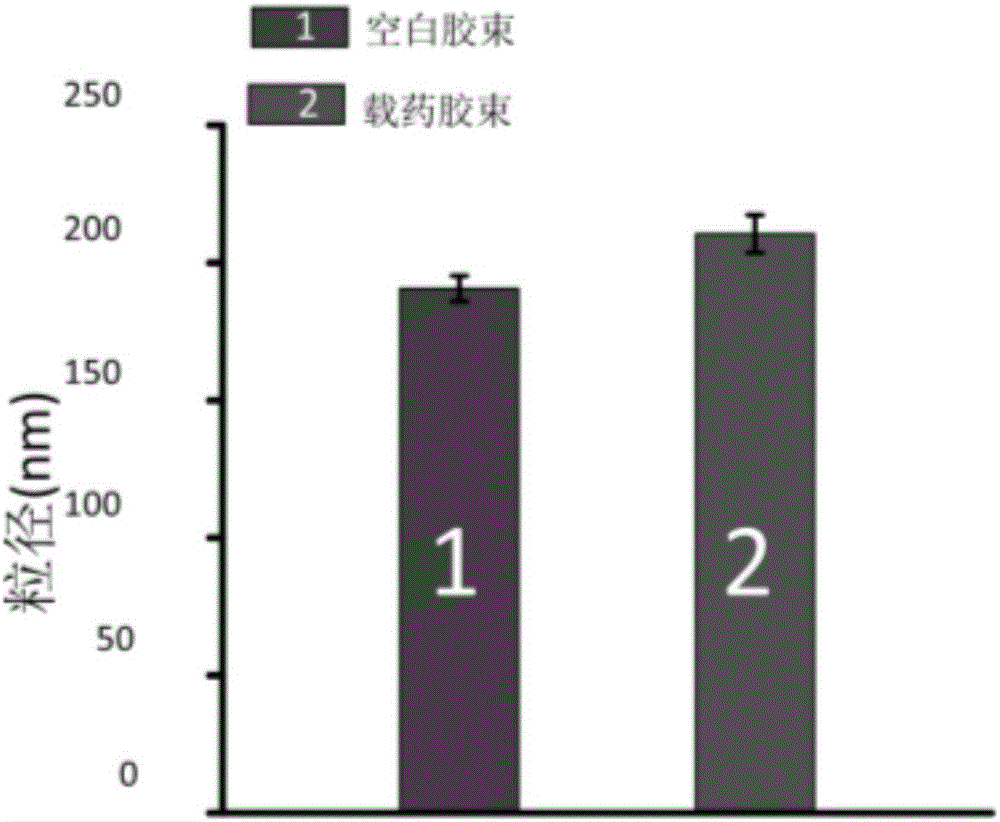
The invention discloses an amphiphilic chitosan derivative drug-loaded nano-micelle and a preparation method and belongs to the field of pharmaceutical preparations. Amphiphilic chitosan derivatives form the micelle by means of self assembling, vitamin E succinate serves as a kernel, and thiolated chitosan serves as a shell; the hydrophobic kernel is used for entrapping a hydrophobic anti-HIV drug, the shell carrying positive charges is used for adsorbing cytomembrane, the loaded drug can be stably released in vivo, the hydrophilic shell is used for enhancing the solubility of the micelle and maintaining the stability of the micelle in a solution, and then the blood circulation time of the micelle after systematic administration is prolonged. The micelle can stably exist in all kinds of body fluid for more than 10 hours after particles are formed, the particle size is smaller than 200 nanometers, and it is beneficial for controlling the micelle particles to enter cells without being removed by macrophages. The delivery system can keep stability under the condition that serums exist, the drug is delivered into the cells, and good biocompatibility is achieved.
Owner:BEIJING UNIV OF TECH
Gynostemma pentaphylla buccal tablet and preparation method thereof
The invention provides a gynostemma pentaphylla buccal tablet and a preparation method thereof. The gynostemma pentaphylla buccal tablet comprises the following components by weight percent: 10-20% of gynostemma pentaphylla ultra-fine powder, 70-80% of mannitol, 2-6% of xylitol, 1-3% of ethanol, 0.1-0.3% of menthol, 3-5% of magnesium stearate and 0.1-0.3% of chitosan, wherein the particle size of the gynostemma pentaphylla ultra-fine powder is 1500-1800 meshes. The preparation method comprises the following steps: preparing a filler, preparing a soft material, pelleting, finishing granules, tabletting, sterilizing, packaging and the like. The gynostemma pentaphylla in the product exists in a form of ultra-fine powder; the fragrance and mouthfeel of the gynostemma pentaphylla can be maintained to the greatest extent, the effective components of the gynostemma pentaphylla can be favorably absorbed in an oral cavity; and the health-care effect of the product is greatly enhanced. The product is good in mouthfeel, has unique flavor of the gynostemma pentaphylla, a smooth surface, consistent color and luster, good hardness and good slaking characteristic, and is suitable for middle aged and elderly people, as well as people with hyperlipidemia, hyperglycemia and obesity.
Owner:李嘉
Application of lead-contaminated soil restoring fixing agent synthesized based on microorganism assembly
InactiveCN105647539ALarge specific surface areaHigh reactivityAgriculture tools and machinesOrganic fertilisersMicroorganismNano hydroxyapatite
The invention discloses application of a lead-contaminated soil restoring fixing agent synthesized based on microorganism assembly. Nano-hydroxyapatite serves as a central substance and wrapped with aspergillus niger hyphae, and the lead-contaminated soil restoring fixing agent is prepared, so that the problem that a powder material is excessively scattered in practical application is solved. The synthesized product is mixed with soil to be uniform according to a certain proportion, fixing is conducted for 7 days, and the available lead in the soil is reduced by 42.8%-48.6%. The fixing agent is simple in preparation process, low in cost, efficient, free of poisons, biodegradable and free of secondary contamination and is an environment-friendly fixing agent.
Owner:CENT SOUTH UNIV
Acidic buffering sterilization gel for vagina gynaecologically
ActiveCN102266283BMaintain moistureEfficient killingPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicineGlycerol
The invention discloses an acidic buffering sterilization gel for vagina gynaecologically. The gel consists of the following substances in parts by weight: 0.2-0.5 part of bactericide, 12-12.5 parts of glycerol, 83-84 parts of water, 1-1.5 parts of Carbomer, 1.5-2 parts of polycarbophil, 0.2-0.3 part of triethanolamine, 0.01-0.03 part of vitamin E, and 0.01-0.03 part of mint. The acidic bufferingsterilization gel has the advantages that: pathogenic bacteria can be quickly and effectively killed, inflammatory symptoms can be quickly and effectively relieved, normal pH value of the vagina is maintained, elasticity of the inner wall in the vagina can be kept, wetness of the vagina is maintained, and drug tolerance and drug resistance do not generate.
Owner:石家庄诺利达医疗器械有限公司
Thermosensitive in-situ gel preparation for norcantharidin injection and preparation method and application of thermosensitive in-situ gel preparation
ActiveCN105902485APromote formationEasy to administerAerosol deliveryOintment deliveryGel preparationPolyethylene glycol
The invention discloses a thermosensitive in-situ gel preparation for norcantharidin injection and a preparation method and an application of the thermosensitive in-situ gel preparation. The thermosensitive in-situ gel preparation is prepared from a solvent and solute, wherein the solute is prepared from the following components by weight-to-volume ratio: 0.0025g / mL of norcantharidin, 0.19g / mL of poloxamer 407, 0.004g / mL of poloxamer 188, 0.0057g / mL of polypropylene, 0.0019g / mL of polyethylene glycol, 0.0008g / mL of hydroxypropyl methyl cellulose and 0.0002g / mL of chitosan. The gel temperature of the in-situ gel preparation is 34 DEG C, is close to the body temperature of a human body, and accords with the requirements of in vivo gel for injection; gel corrosion and drug release are carried out at a similar velocity; the drug is controlled to be slowly released by gel corrosion; the thermosensitive in-situ gel preparation has good drug slow-release and sustained-release effects; and the medication safety is good.
Owner:FIRST PEOPLES HOSPITAL OF YUHANG DISTRICT HANGZHOU
Polygonatum odoratum buccal tablet and production method thereof
InactiveCN103446435AKeep the scentKeep the tasteAntibacterial agentsMetabolism disorderAcute hyperglycaemiaDisease
The invention provides a polygonatum odoratum buccal tablet and a production method thereof. The polygonatum odoratum buccal tablet comprises the following components in parts by weight: 8-15% of polygonatum ordoratum submicron powder, 30-40% of mannitol, 33-45% of microcrystalline cellulose, 2-6% of xylitol, 1-2% of ethyl alcohol, 0.1-0.3% of menthol, 3-5% calcium sulfate and 0.1-0.3% chitosan, wherein the granularity of the polygonatum ordoratum submicron powder is 1600-1700 meshes. The production method comprises the following steps: preparing a filler, manufacturing a soft material, prilling, gathering granules, tabletting, sterilizing, and packing and the like. According to the invention, the polygonatum ordoratum can exist in the form of submicron powder, so that the fragrance and taste of the polygonatum ordoratum can be retained to the greatest extent, the effective constituent of the polygonatum ordoratum can be better absorbed inside an oral cavity, and the health care effect of products can be greatly enhanced. The polygonatum ordoratum bucaal tablet has the characteristics of being good in taste, smooth in surface, conform in color and luster, good in hardness, better in disintegrative characteristic and applicable to being taken by the middle-aged and aged people, and people with diseases of hyperlipidemia, hyperglycemia and obesity.
Owner:李嘉
Ultra porous hydrogel complex substance, preparing method and use in pharmaceutics thereof
InactiveCN1253147CEasy to manufactureRapid swellingPeptide/protein ingredientsPharmaceutical delivery mechanismFoaming agentCrosslinked polymers
The invention discloses a superporous hydrogel composite, its preparation method and its application in pharmacy. The superporous hydrogel composite of the present invention contains a crosslinked polymer and carbomer, and has a superporous structure. The polymer is formed by polymerization of at least one unsaturated ethylenic monomer and a polyene crosslinking agent. The superporous hydrogel composite of the present invention is prepared by the following steps: mixing at least one unsaturated ethylenic monomer, a polyene crosslinking agent, carbomer and a foaming agent; Formation of superporous hydrogel composites under the condition of bubbles. The superporous hydrogel complex of the present invention can be used in gastric floating preparations and protein polypeptide oral drug delivery systems. Compared with the prior art, it has bioadhesive properties and protease inhibition.
Owner:FUDAN UNIV
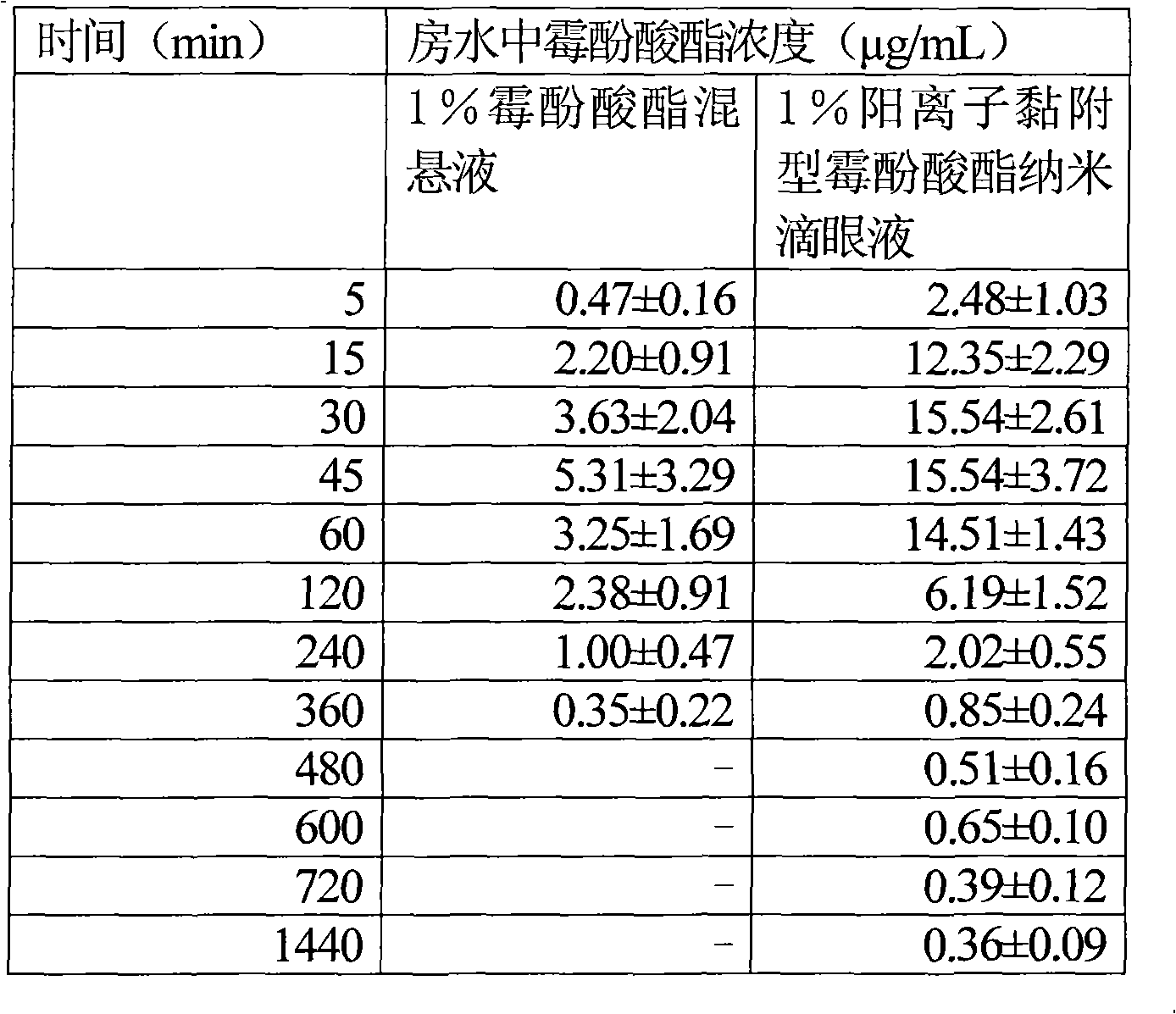
Cationic adhesion type mycophenolate mofetil nano eye drop
InactiveCN101590015AImprove solubilityIncreased corneal resorption featuresOrganic active ingredientsSenses disorderSolubilityPhospholipid
The invention relates to a cationic adhesion type mycophenolate mofetil nano eye drop, which comprises mycophenolate mofetil of which the mass percentage concentration is 1 percent, proper amount of osmotic pressure modifying agent and pH modifying agent. The eye drop is characterized in that the eye drop also comprises chitosan, Pluronic F-68 and phospholipid, wherein the mass percentage concentration of the phospholipids in the eye drop is between 0.2 and 2 percent; the mass percentage concentration of the Pluronic F-68 in the eye drop is between 0.5 and 4 percent; the mass percentage concentration of the chitosan in the eye drop is between 0.5 and 4 percent; the molecular weight of the chitosan is between 5,000 and 300,000; the deacetylation degree is more than 85 percent; and the grain diameter of the mycophenolate mofetil is between 10 and 700 nm. The eye drop has the characteristics that: the mycophenolate mofetil with nanometer grain diameter improves the medicament solubility, the safety and the effectiveness; mycophenolate mofetil medicament particles are charged with positive charges so as to be favorable for improving the cornea absorption of the medicament, improving the medicament effect, prolonging the medicament duration, and greatly improving the cornea adhesion.
Owner:SHANDONG EYE INST
A kind of iron-based biochar material and its preparation and application
ActiveCN105733588BImprove adsorption capacityAvoid the defects that are easy to flow away with waterContaminated soil reclamationOrganic fertilisersCarbonizationAspergillus niger
Owner:CENT SOUTH UNIV
Ecological breeding feed for mutton sheep and preparation method of ecological breeding feed
The invention discloses an ecological breeding feed for mutton sheep and a preparation method of the ecological breeding feed, and belongs to the technical field of feed preparation. The ecological breeding feed comprises the following raw materials of alfalfa, corn germ meal, distillers' grains DDGS, corn, middling, rice bran, bran, soybean meal, rapeseed meal, a premix feed, a microecological preparation, an additive A, an additive B and an additive C. The ecological breeding feed for mutton sheep is prepared through the steps of crushing materials, performing mixing, performing granulation,performing drying and the like. Through adoption of the prepared ecological breeding feed for mutton sheep, the average daily feed intake and the average daily gain of the mutton sheep and the digestibility of each nutrient substance can be effectively and obviously increased, and the prevalence rate can be reduced.
Owner:DAXIN SCI & TECH INFORMATION RES INST
Composite denitrification biological tank
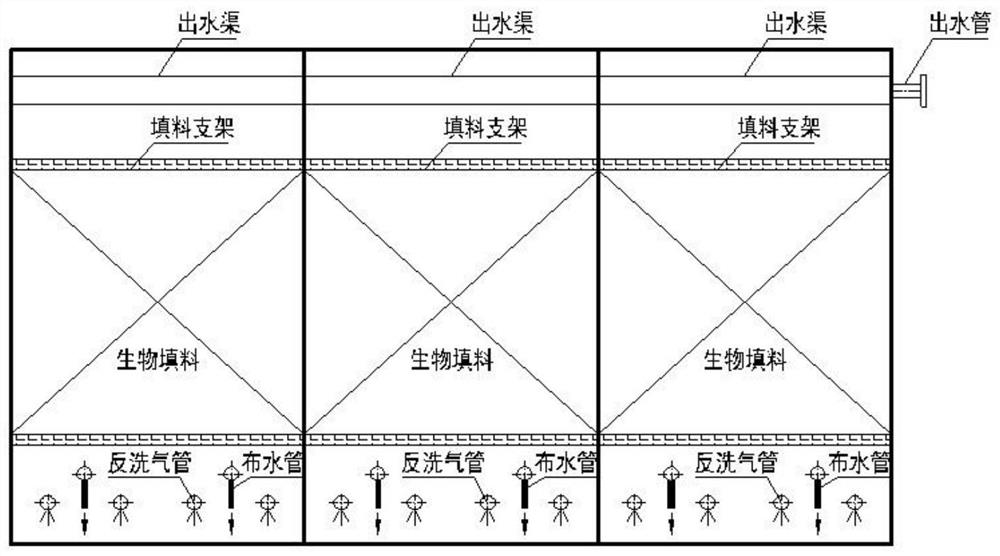
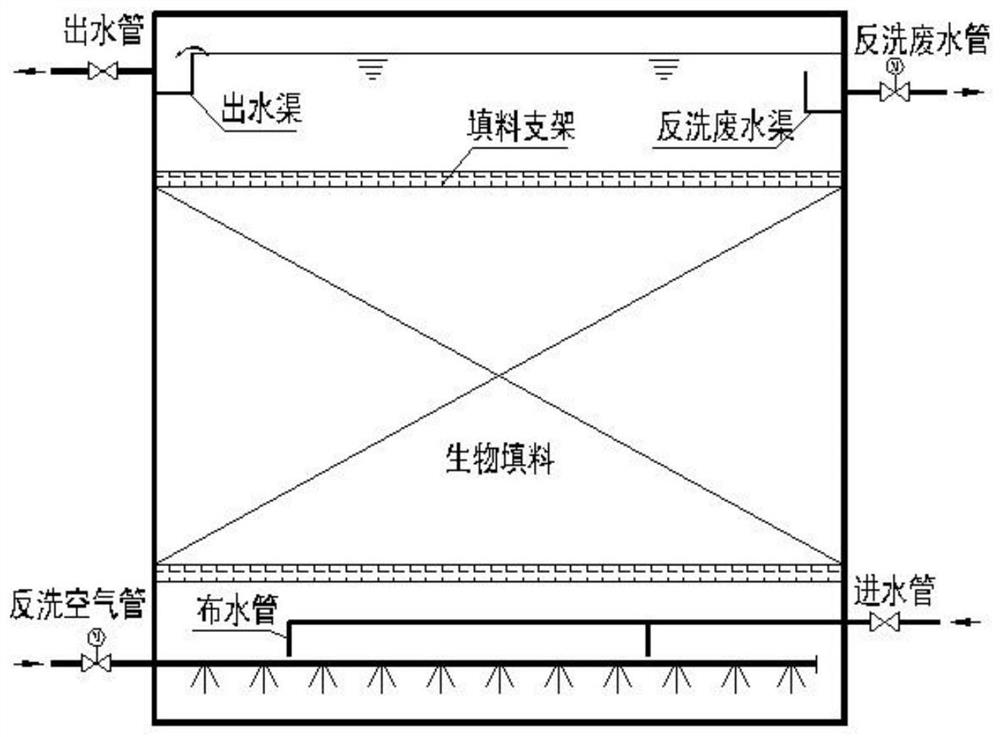
PendingCN113135616AAchieve removalHigh removal rateSpecific water treatment objectivesTreatment with aerobic and anaerobic processesWastewaterDrainage tubes
The invention discloses a composite denitrification biological tank which comprises a biological tank body, and the biological tank body is composed of a water distribution system, a gas backwashing system, a biological filler, a filler support, a backwashing wastewater drainage channel, a backwashing wastewater drainage pipe and a carbon source accurate adding control system. The water distribution system comprises a water inlet pipe, water distribution pipes, a water outlet pipe and a water outlet channel, the water inlet pipe communicated with the biological tank body is installed on one end face of the bottom of the biological tank body, the water distribution pipes are evenly arranged on the water inlet pipe located in the biological tank body at equal intervals, and a water outlet channel is arranged on the upper portion of the biological tank body. The water outlet pipe is mounted at the side end of the upper part of the biological tank body, is distributed away from one side of the water inlet pipe and can realize fluid communication with the water outlet channel; and the air backwashing system comprises a backwashing air pipe and an air inlet control valve, and the backwashing air pipe is mounted on one side, deviating from the water inlet pipe, of the biological tank body. Thecomposite denitrification biological tank has the advantages of high efficiency, stability, simplicity in management, low cost and low energy consumption.
Owner:北京弘昇达环境科技有限公司
Calcium alginate composite microspheres for stabilizing water-in-oil type Pickering emulsion and preparation method thereof
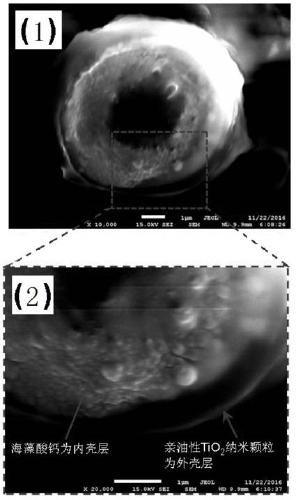
ActiveCN106674555BBiocompatibleBioadhesiveCosmetic preparationsToilet preparationsMicrosphereBiocompatibility Testing
The invention discloses calcium alginate composite microspheres for stabilizing water-in-oil type Pickering emulsions and a preparation method thereof, belonging to the technical field of material polymers. Alkylsilane-grafted inorganic nanoparticles are coated on the surface of calcium alginate microspheres to form calcium alginate composite microspheres. The particle size distribution of the microspheres is 2-8 μm; it has a core-shell structure, the core is hollow, and the shell has two layers. The total thickness of the two-layer shell is 1~1.5µm, calcium alginate is the inner shell, and inorganic nanoparticles are assembled to form the outermost shell; it overcomes the fact that chitosan coated on the surface of calcium alginate microspheres can only stabilize the oil-in-water type Pickering emulsion, as a drug carrier, can only encapsulate oil-soluble drugs but not water-soluble drugs. The use of improved emulsion polymerization to prepare calcium alginate composite microspheres also has the biocompatibility of calcium alginate, Adhesion and encapsulation of protein macromolecules can stabilize the water-in-oil Pickering emulsion very well. It can widely expand the application of Pickering emulsion as a drug carrier in the fields of biomedicine, cosmetics industry, food industry and enzyme engineering.
Owner:SOUTHWEST JIAOTONG UNIV
Gel ointment for treating flat wart
InactiveCN101524400ABioadhesiveGrowth inhibitionAerosol deliveryOintment deliveryAdditive ingredientCurative effect
The invention relates to a gel ointment for treating flat wart. Carboxymethylcellulose sodium and other gels are compounded to form an ointment substrate. The carried medicaments comprise oleum fructus bruceae, gentian, isatidis root and gromwell. The gel ointment has the advantages that: 1, the ointment has function of inhibiting the growth of fungus; 2, due to the wrapping effect of a gel carrier, the medicaments are milder and avoid corrosive effect on the peripheral skin of a wart body; 3, the gel ointment can slowly and lastingly release the medical compositions, thereby improving the curative effect; 4, the ointment has biological adhesion so that the gel ointment is easy to adhere on an affected part; and 5, the gel ointment is more convenient to use.
Owner:大连永兴生物医药孵化器有限公司
Cerebral aneurysm micro-occlusion device
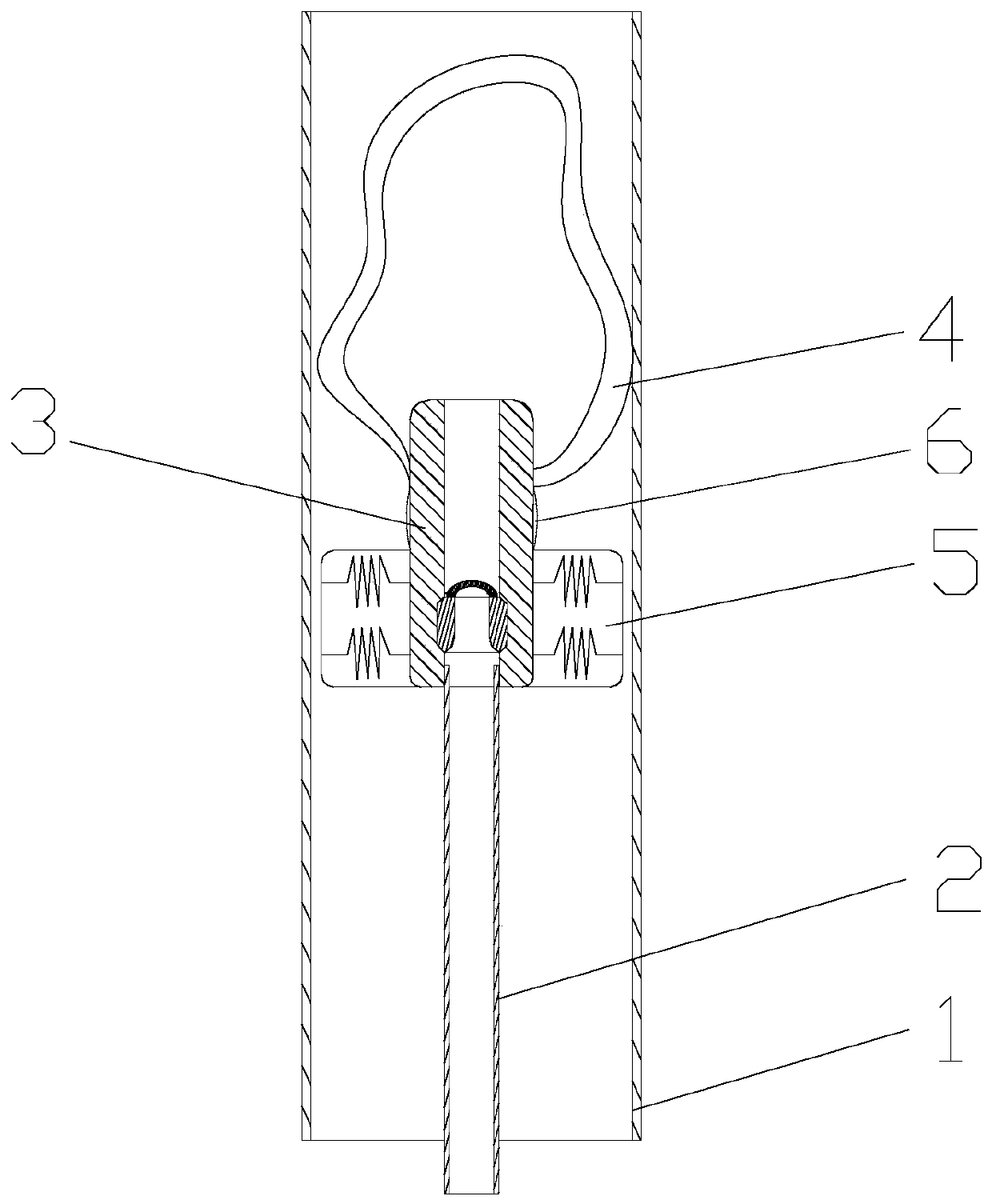
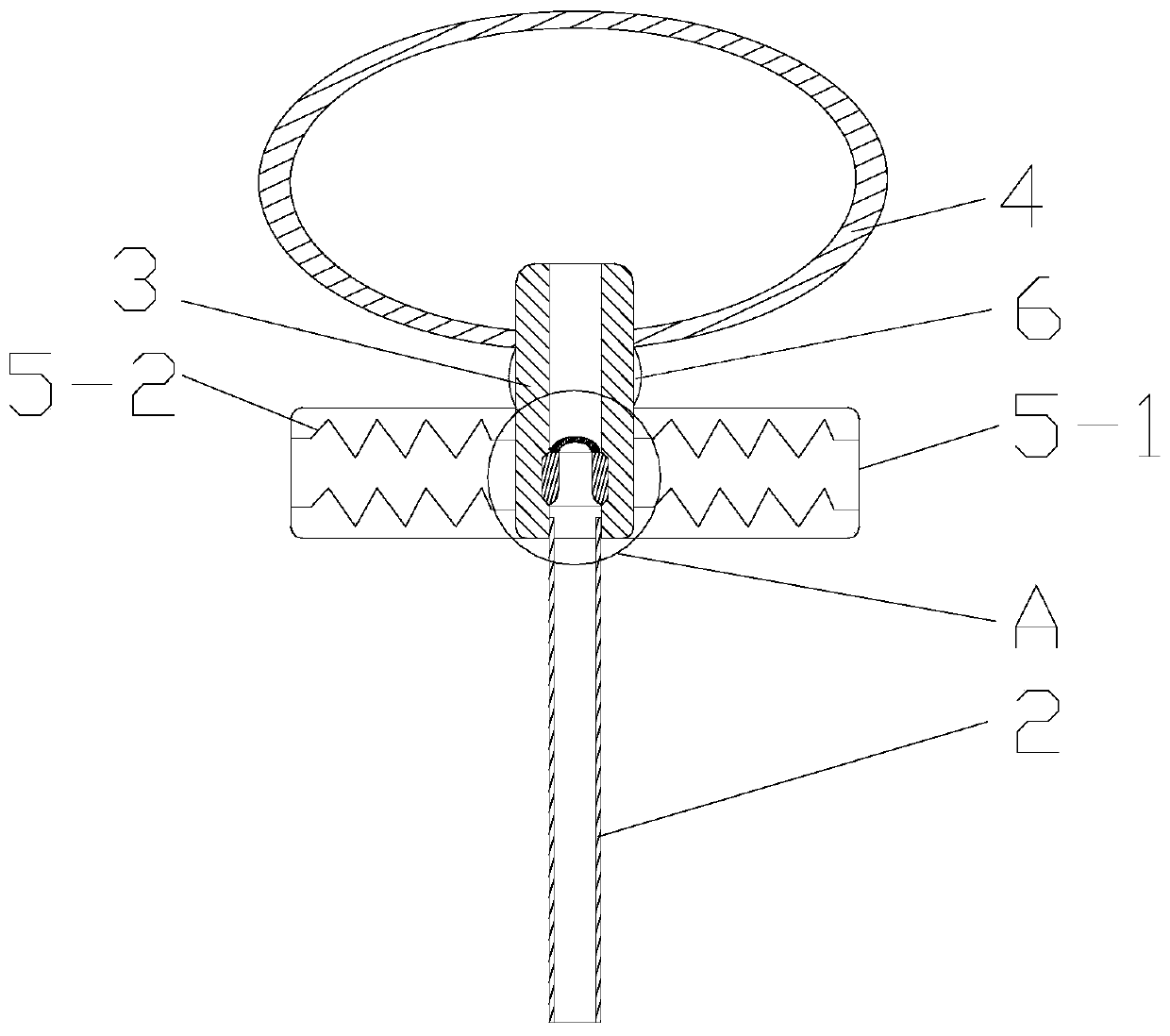
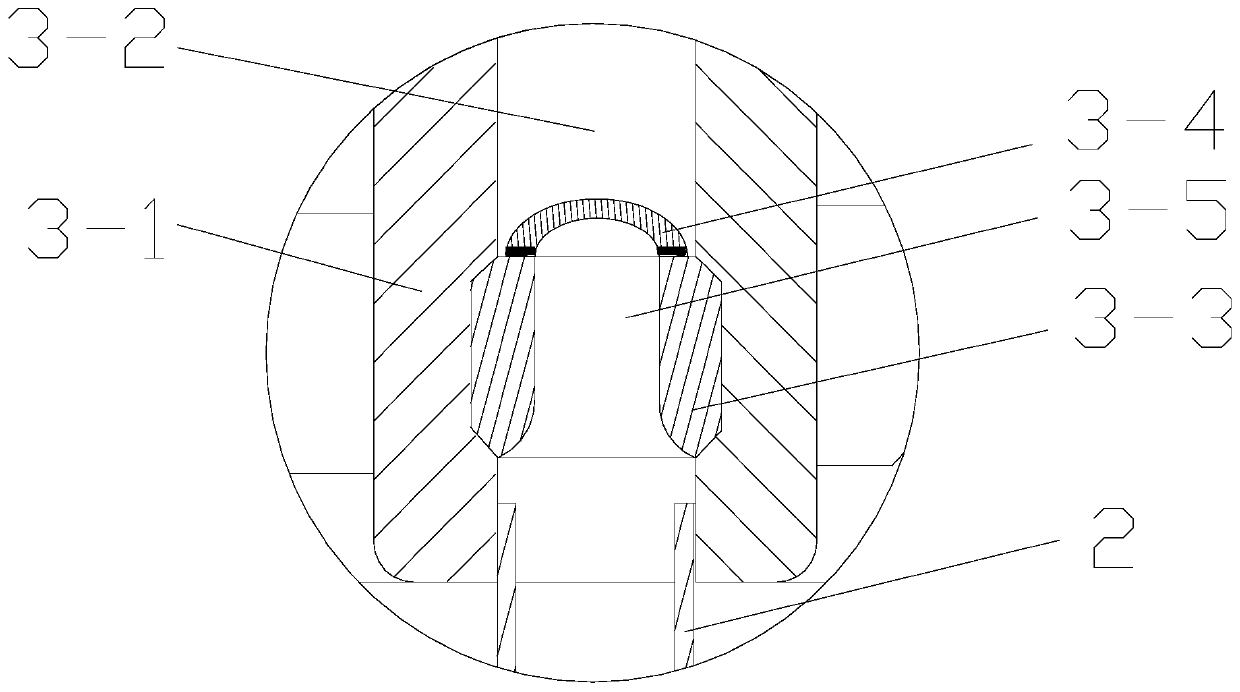
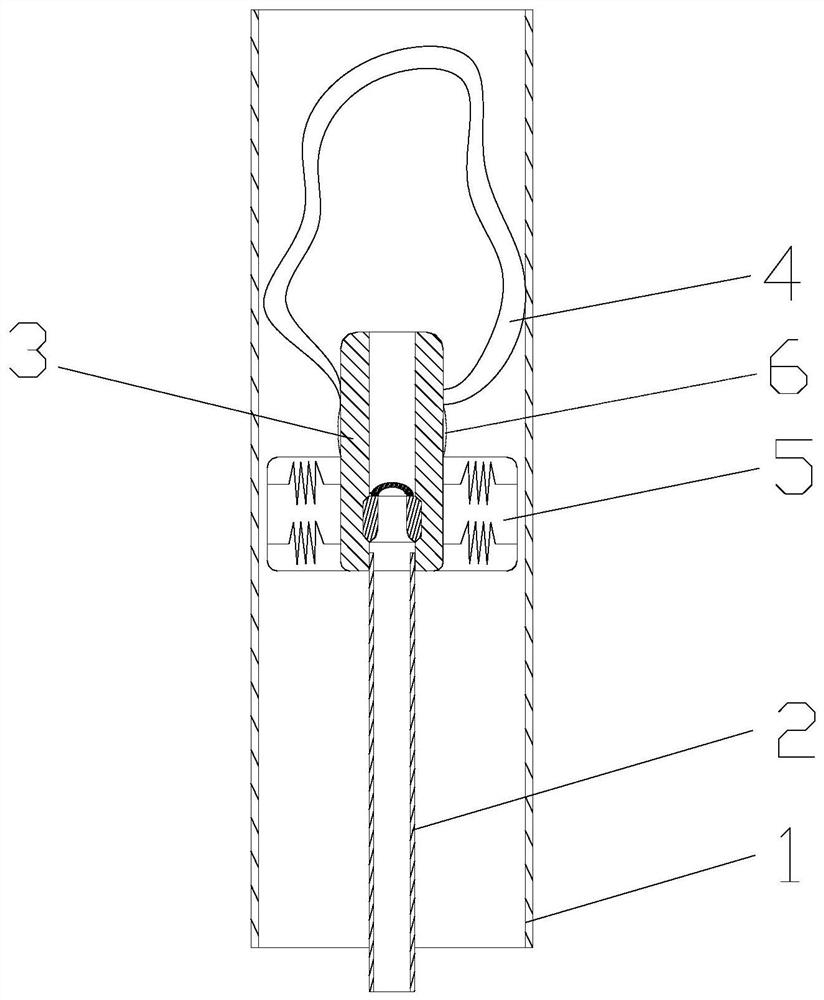
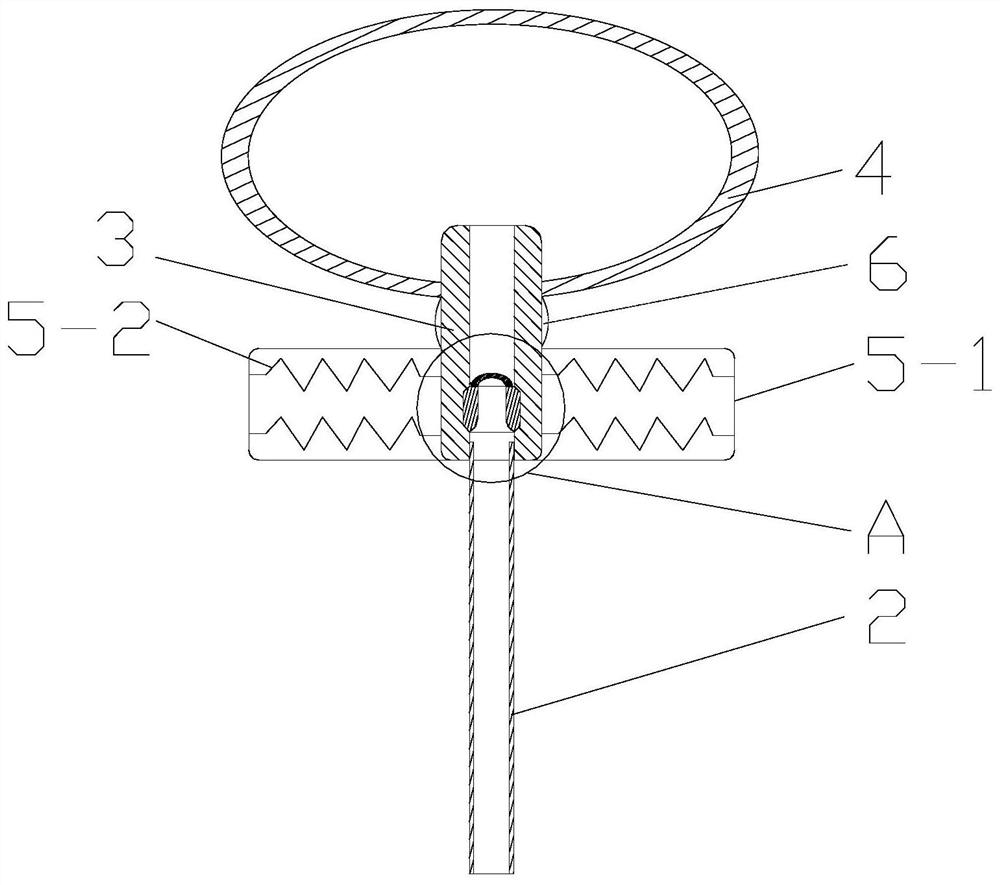
ActiveCN109998621ASimple structureReasonable designSurgical navigation systemsOcculdersCatheterRupture risk
The invention discloses a cerebral aneurysm micro-occlusion device. The cerebral aneurysm micro-occlusion device comprises a main occlusion device body, a filling liquid, a conveying pipe and a pushing catheter, wherein the main occlusion device body comprises a check valve; a liquid inlet end of the check valve sleeves a limit block; a liquid outlet end of the check valve sleeves a fluid blockingbody; the fluid blocking body comprises a biological gel material with elastic deformation performance; the filling liquid enters the fluid blocking body through the pushing catheter and the check valve. The components of the device have small dependence on the process size and are particularly applicable to micro cerebral aneurysms. The filling liquid enters the fluid blocking body through the pushing catheter and the check valve, so that the biological gel material with the elastic deformation performance is elastically deformed under the filling action of the filling liquid, and correspondingly the biological gel material expands and plays a role in blocking a cerebral aneurysm. The biological gel material is selected for making the fluid blocking material, has biological adhesion andcan successfully adhere to the cerebral aneurysm, so that the strength of the cerebral aneurysm is enhanced, and the rupture risk of the cerebral aneurysm is reduced.
Owner:WUHAN VICKOR MEDICAL TECH CO LTD
Micro-occluder for cerebral aneurysm
ActiveCN109998621BSimple structureReasonable designSurgical navigation systemsOcculdersMicrocomputerCatheter
The invention discloses a micro-occluder for cerebral aneurysm, which comprises a main body of the occluder, a filling liquid, a delivery tube, and a push catheter. A limit block is connected, and the liquid outlet end of the check valve is provided with a blocking body, the blocking body is made of biogel material with elastic deformation properties, and the filling liquid passes through the push conduit and the check valve Enter the bluff body. The parts of the invention have less dependence on the process size, and are especially suitable for small cerebral aneurysms. The filling fluid enters the blocking body through the push catheter and the check valve, so that the biogel material with elastic deformation performance can be used during the filling of the filling fluid. Elastic deformation occurs under the action, so as to expand and expand, and play the role of blocking cerebral aneurysms. The material of the blocking fluid is biogel material, which has bioadhesiveness and can successfully adhere to cerebral aneurysms, thereby Increase the strength of brain aneurysms and reduce the risk of brain aneurysm rupture.
Owner:WUHAN VICKOR MEDICAL TECH CO LTD
Refreshing and moisturizing body lotion
PendingCN107714612APlay the role of beauty and skin careGood biocompatibilityCosmetic preparationsToilet preparationsBiotechnologyCarrageenan
The invention discloses a refreshing and moisturizing body lotion, belonging to the technical field of daily chemical products. The body lotion developed by the invention contains serum protein hydrolysate, bacterial cellulose, animal bone ash, an emulsifier, Carbomer, snail mucus, a thickener, taurine, nicotinamide, vitamins, 1,2-propylene glycol, plant essential oil and water, wherein the serumprotein hydrolysate is prepared by the steps of carrying out constant-temperature stirring enzymolysis on serum proteins, trypsin and water, and carrying out enzyme deactivation; the animal bone ash is prepared by the steps of crushing cow bones, and calcining with sepiolite; the bacterial cellulose is prepared by the steps of culturing acetobacter xylinum in a culture medium for 7 days, taking out, and generating a layer of gel film on the surface of the culture medium; the emulsifier is prepared by mixing non-hydrated phospholipid with tea saponin; and the thickener is prepared by mixing carrageenan, peach gum and xanthan gum. The prepared refreshing and moisturizing body lotion is light and thin in texture and has an excellent long-acting moisturizing effect.
Owner:吴迪
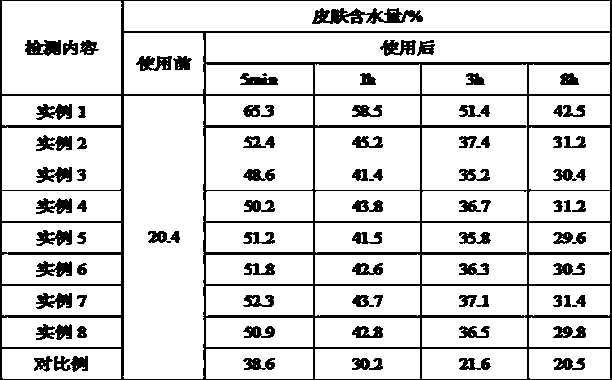
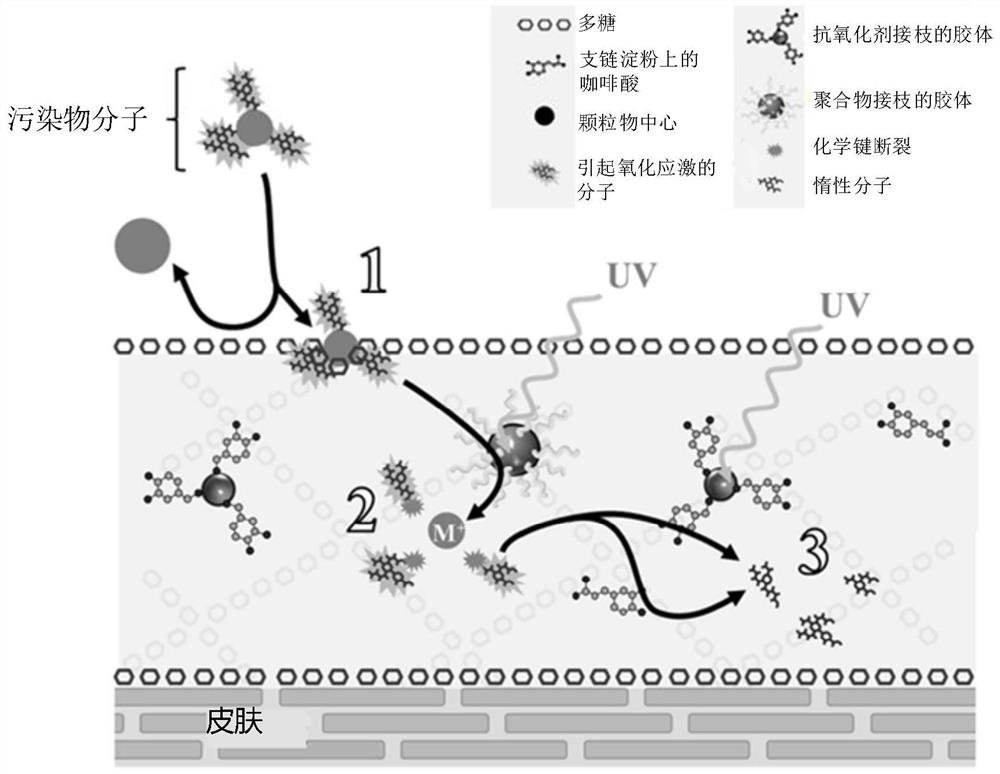
Method for topical protection against atmospheric pollutant molecules and/or free radicals produced by exposure to ultraviolet radiation
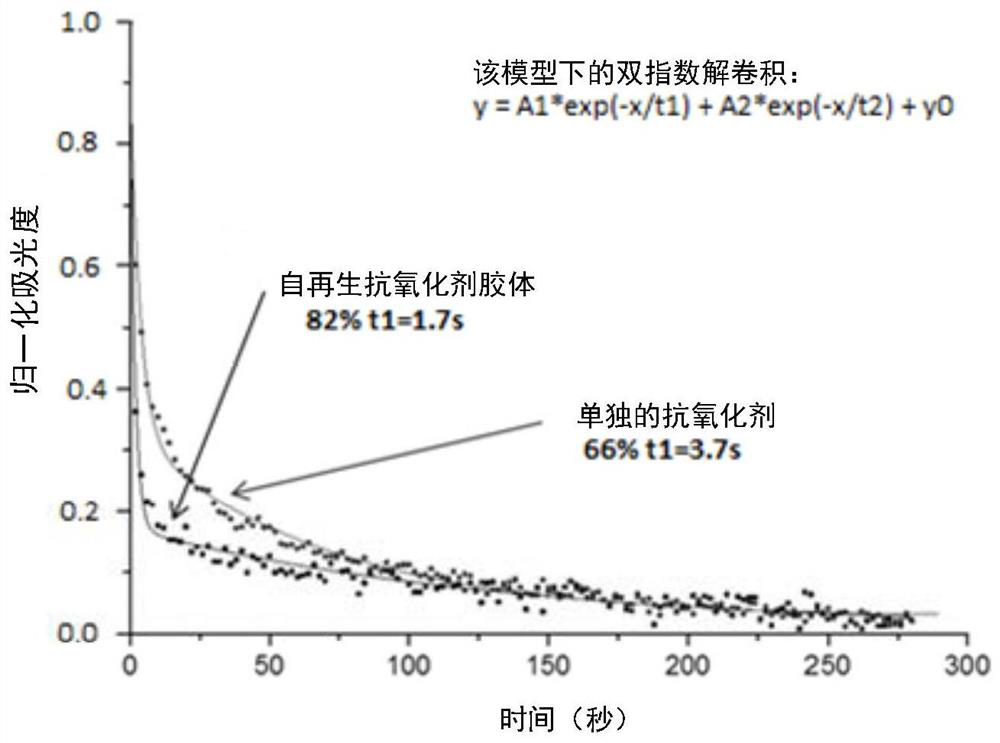
PendingCN112512641APropose anti-pollution "barrier" effectEliminate oxidationCosmetic preparationsToilet preparationsPhotocatalytic degradationPhoto catalysis
Disclosed is a method for topical protection against atmospheric pollutant molecules and ultraviolet (UV) radiation, comprising the steps of: a) producing a polymer matrix which is both repellent andanti-adhesive to atmospheric pollutant molecules, by means of a first biocompatible polymer (PB1); b) under the effect of the UV radiation, photocatalytically degrading the pollutant molecules havingpenetrated the polymer matrix by means of first semiconductor colloids (Col-1) onto which a second biocompatible polymer (PB2) has been grafted, which results in the formation of free radicals; c) neutralizing said free radicals by means of at least 2 antioxidants, which are: a first antioxidant in the form of second semiconductor colloids (Col-2) onto which the first antioxidant (AntiOx-1) has been covalently grafted, said second grafted colloids (Col-2) auto-regenerating under the effect of the UV radiation, and a second antioxidant agent (AntiOx-2) that is not present in the form of colloids grafted with an antioxidant; and d) stabilizing the polymer matrix by means of the second antioxidant agent (AntiOx-2).
Owner:比奥尼克莱公司
A temperature-sensitive in-situ gel preparation for norcantharidin injection and its preparation method and application
ActiveCN105902485BPromote formationEasy to administerAerosol deliveryOintment deliveryGel preparationPolyethylene glycol
The invention discloses a thermosensitive in-situ gel preparation for norcantharidin injection and a preparation method and an application of the thermosensitive in-situ gel preparation. The thermosensitive in-situ gel preparation is prepared from a solvent and solute, wherein the solute is prepared from the following components by weight-to-volume ratio: 0.0025g / mL of norcantharidin, 0.19g / mL of poloxamer 407, 0.004g / mL of poloxamer 188, 0.0057g / mL of polypropylene, 0.0019g / mL of polyethylene glycol, 0.0008g / mL of hydroxypropyl methyl cellulose and 0.0002g / mL of chitosan. The gel temperature of the in-situ gel preparation is 34 DEG C, is close to the body temperature of a human body, and accords with the requirements of in vivo gel for injection; gel corrosion and drug release are carried out at a similar velocity; the drug is controlled to be slowly released by gel corrosion; the thermosensitive in-situ gel preparation has good drug slow-release and sustained-release effects; and the medication safety is good.
Owner:FIRST PEOPLES HOSPITAL OF YUHANG DISTRICT HANGZHOU
Disease-resistant type feed additive capable of promoting digestion and absorption and application of disease-resistant type feed additive
InactiveCN108813174AIncrease average daily feed intakeIncrease daily weight gainFood processingAnimal feeding stuffAnimal sciencePumpkin seed
The invention discloses a disease-resistant type feed additive capable of promoting digestion and absorption and an application of the disease-resistant type feed additive, and belongs to the technical field of feed additives. The disease-resistant type feed additive capable of promoting digestion and absorption comprises the following raw materials of licorice roots, medicated leaven, endotheliumcorneum gigeriae galli, pumpkin seeds, azedarach, pseudobulbus cremastrae seu pleiones, umbellate rockjasmine herbs, rhizoma fagopyri cymosi roots, carboxymethyl-beta-cyclodextrin and sodium polyacrylate. The disease-resistant type feed additive capable of promoting digestion and absorption is applied to an ecological breeding feed for mutton sheep; after the ecological breeding feed for mutton sheep, prepared from the disease-resistant type feed additive capable of promoting digestion and absorption, is used, the average daily feed intake and the average daily gain of the mutton sheep and the digestibility of each nutrient substance can be effectively increased, and the prevalence rate can be reduced.
Owner:DAXIN SCI & TECH INFORMATION RES INST
Cationic adhesion type mycophenolate mofetil nano eye drop
InactiveCN101590015BImprove solubilityIncreased corneal resorption featuresOrganic active ingredientsSenses disorderSolubilityPhospholipid
Owner:SHANDONG EYE INST
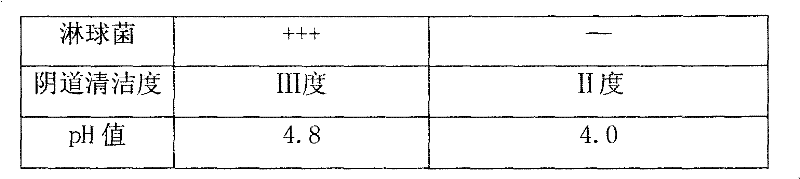
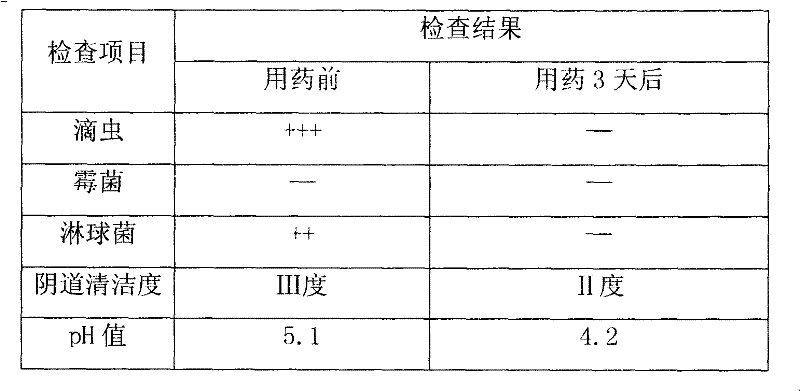
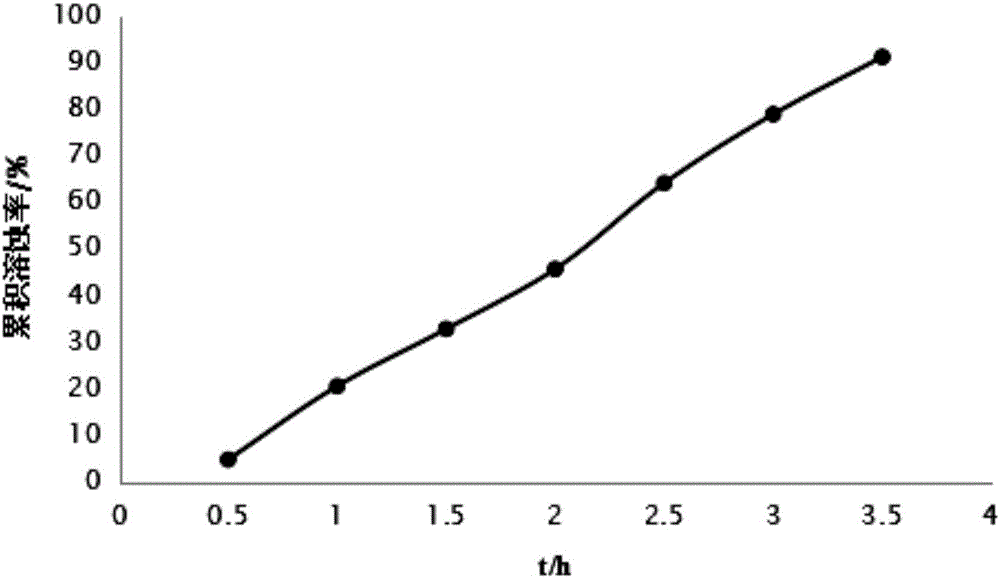
Nifuratel vaginal tablet and preparation method thereof
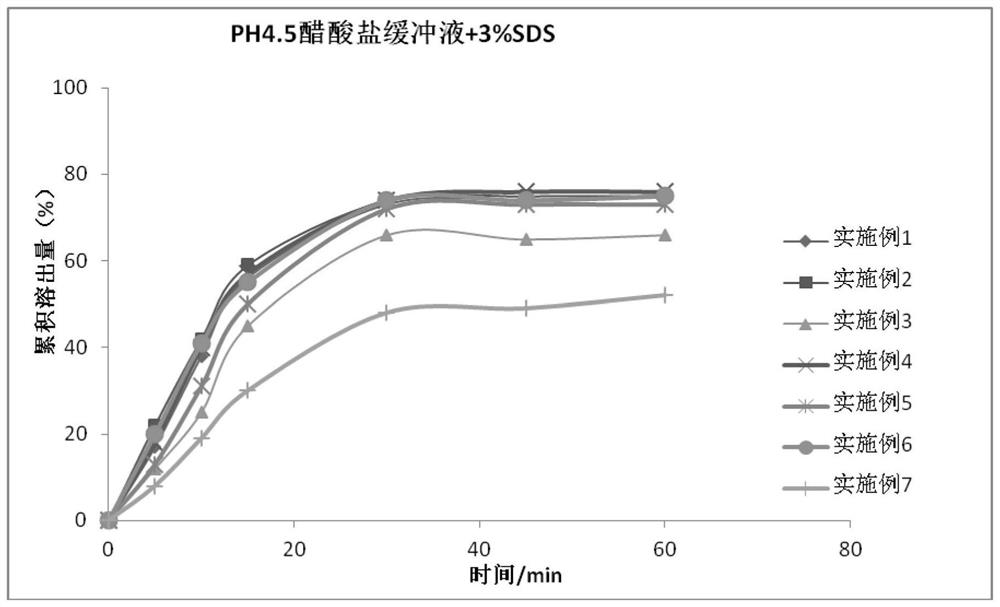
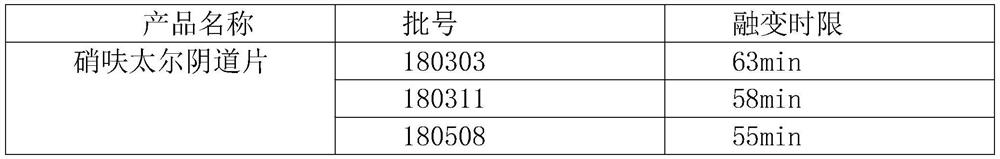
PendingCN112353772AIncrease dissolution ratePlay a sustained release effectOrganic active ingredientsPill deliveryClinical efficacyTherapeutic effect
The invention discloses a nifuratel vaginal tablet, which comprises the following components in parts by weight: 0.2-0.6 part of carbomer, 0.1-0.3 part of alginate and 1 part of nifuratel, wherein theparticle size distribution D90 of micronized nifuratel is 30-60 [mu]m. The invention also discloses a preparation method of the nifuratel vaginal tablet. According to the nifuratel vaginal tablet, the dissolution rate of the nifuratel vaginal tablet is increased by controlling nifuratel particle size distribution, the carbomer and the alginate play a role in slow release and have certain biological adhesion, so that the nifuratel vaginal tablet takes effect continuously and quickly and is not prone to falling off from the vagina, the treatment effect is guaranteed, and clothes and trousers are not polluted; and the nifuratel vaginal tablet provided by the invention is convenient to use, quick to take effect, lasting in effect and good in clinical curative effect.
Owner:BEIJING CHENG JI PHARMA
Cation adhesion type natamycin nano eye drops
ActiveCN101385703BImprove solubilityIncrease corneal resorptionOrganic active ingredientsSenses disorderSolubilityPhospholipid
A cationic adhesive-type natamycin nanometer eye drop comprises natamycin, a proper amount of osmoregulator and pH regulator. The eye drop is characterized by further comprising chitosan, pluronic F-68 and phospholipid, wherein, the mass percent concentration of the phospholipid in the eye drop is 0.01-2%, the mass percent concentration of the pluronic F-68 in the eye drop is 0.1-20%, and the masspercent concentration of the chitosan in the eye drop is 0.01-4%. The molecular weight of the chitosan is 5000-300000, and the deacetylation degree of the chitosan is greater than 85%. The natamycinnanometer eye drop is characterized by making a common natamycin suspension into suspension particles with the diameter ranging from 10nm to 1000nm, thus increasing solubility of the eye drop and enhancing safety and effectiveness. The natamycin particles of the common suspension are modified by the phospholipid and the chitosan to cause the particles to be positively charged, which is helpful toimprove absorption of the eye drop by cornea, enhances drug effect and prolongs the action time of the eye drop. And the chitosan has bioadhesion function, which enhances the adhesiveness between medicinal particles and the cornea.
Owner:SHANDONG EYE INST
A cadmium-contaminated soil remediation fixative based on microbial assembly synthesis and its preparation and application method
ActiveCN105733599BLarge specific surface areaHigh reactivityOrganic fertilisersSoil conditioning compositionsEutrophicationPhosphate
The invention discloses a cadmium-contaminated soil remediation fixing agent based on microorganism assembly synthesis as well as preparation and an application method thereof. At present, multiple nano phosphate fixing agents for cadmium-contaminated soil remediation are easily dispersed and lost, underground water eutrophication is caused, and further secondary pollution is caused. Aiming at solving the problems, nano-hydroxyapatite is taken as a central substance, aspergillus niger hyphae are adopted for wrapping the nano-hydroxyapatite, and a cadmium-contaminated soil remediation fixing agent specific to high contamination and high acidity is prepared, so that the problem of excessive dispersion in practical application is solved. A synthesized product is uniformly mixed with cadmium-contaminated soil in a certain rate, and effective state cadmium content is reduced by 67-86%. The cadmium-contaminated soil remediation fixing agent disclosed by the invention is simple in preparation process, low in cost, efficient, non-toxic and biodegradable and has no secondary pollution, thereby being an environment-friendly fixing agent.
Owner:JIANGXI GAIA ENVIRONMENTAL SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com