Cationic adhesion type mycophenolate mofetil nano eye drop
An adhesive type mycophenolate mofetil and mycophenolate mofetil technology, which is applied in the field of cationic adhesive type mycophenolate mofetil nano eye drops, can solve the problems of serious adverse reactions, limit the application of mycophenolate mofetil, and avoid adverse reactions. , The effect of changing corneal absorption characteristics and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
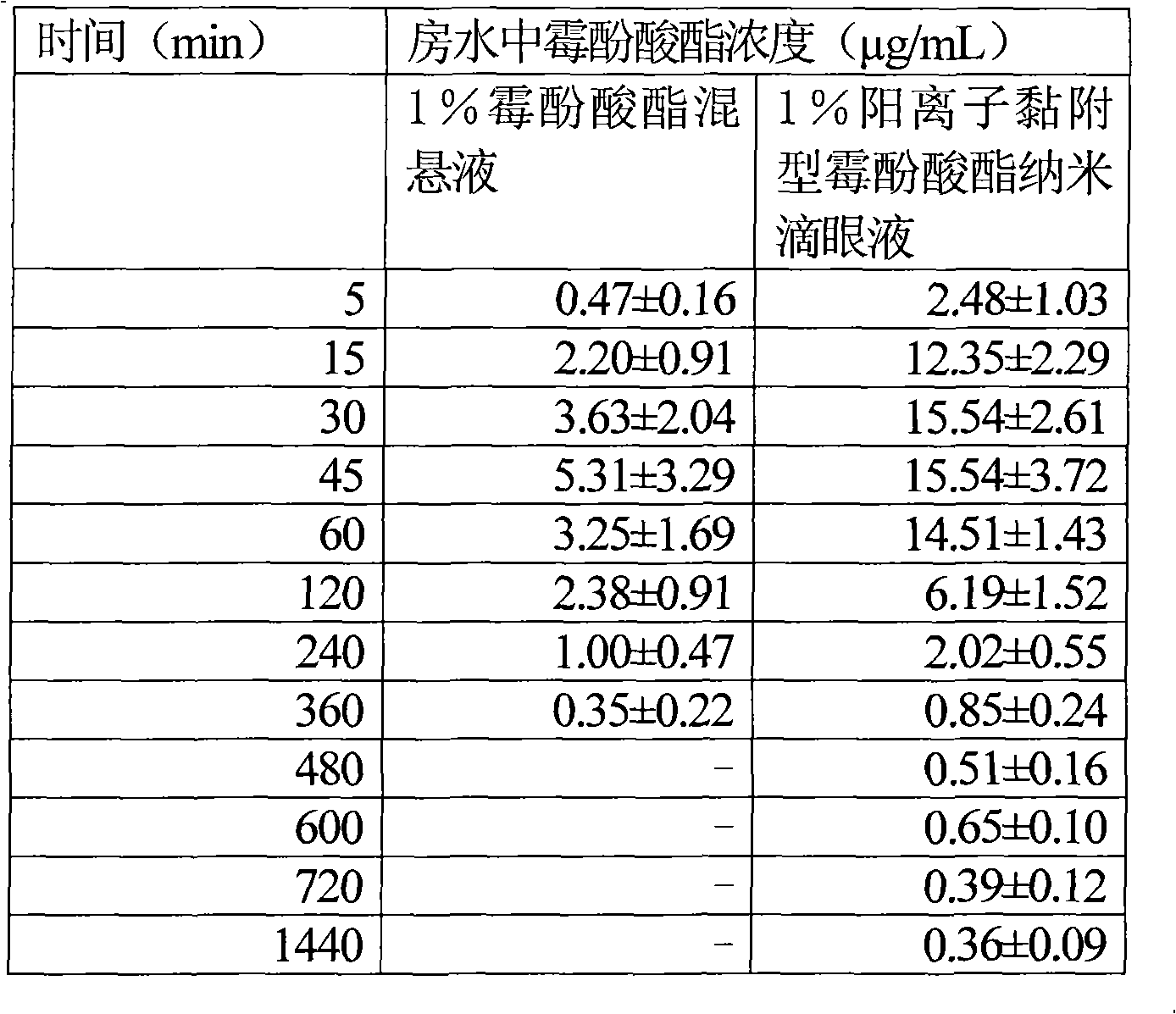
Image
Examples
Embodiment 1
[0020] 0.3g mycophenolate mofetil and 60mg phospholipids were dissolved in 50ml ether, and the ether was fully removed by rotary evaporation to obtain a uniformly dispersed film of medicine and phospholipids, and then 2% chitosan (molecular weight 5000, deacetylation degree 85%) solution 7.5 ml and 7.5ml of 2% F-68 solution, fully wash the membrane, and obtain an emulsion after ultrasonic dispersion, pass the emulsion through a high-pressure homogenizer, first use a pressure of 500bar to circulate 5 times for initial crushing, and then use a pressure of 1500bar to circulate 15 times Further refine and pulverize, then dilute the homogeneous emulsion to 30ml with water for injection, adjust the osmotic pressure to isotonicity with sodium chloride, and dilute the pH value of hydrochloric acid to 6.5 to obtain cationic adhesion type mycophenolate mofetil nanosuspension drops eye drops.
[0021] The particle size range of the nanosuspension measured by the laser particle size poten...
Embodiment 2
[0023] Dissolve 0.3g of mycophenolate mofetil and 0.6g of phospholipid in 50ml of ether, and remove the ether by rotary evaporation to obtain a uniformly dispersed film of medicine and phospholipid, then add 1.2g of chitosan and F -681.2g was dissolved in 25ml of water for injection and fully dissolved, the film was fully washed with the solution of chitosan and F-68, and the emulsion was obtained after ultrasonic dispersion, and the emulsion was passed through a high-pressure homogenizer, and the pressure was circulated for 5 times at 500bar. First pulverize, then use 1500bar pressure to cycle 15 times for further fine pulverization, then dilute the homogeneous emulsion to 30ml with water for injection, adjust the osmotic pressure to isotonic with sodium chloride, and dilute the pH value of hydrochloric acid to 6.5 to obtain cations Adhesive mycophenolate mofetil nanosuspension eye drops. The particle size range of the nanosuspension measured by the laser particle size potent...
Embodiment 3
[0025] The present invention can also be obtained by replacing the chitosan in Example 1 with a chitosan with a molecular weight of 300,000 and a deacetylation degree of 85%. The particle size range of the nanosuspension measured by the optical particle size potential analyzer is 100-700nm, the average particle size is 427nm, and the particle surface potential is +49mV. The obtained nanosuspension was stored at 4° C. for 6 months without light, and the particle size and potential did not change substantially.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com