Nifuratel vaginal tablet and preparation method thereof
A technology of nifuratel and vaginal tablets, which is applied in the field of medicine to achieve the effects of convenient use, long-lasting effect, and improved dissolution rate
Pending Publication Date: 2021-02-12
BEIJING CHENG JI PHARMA
View PDF6 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, there is no technology to solve the above problems of nifuratel vaginal tablets
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0031]
[0032]
Embodiment 2
[0034]
Embodiment 3
[0036]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Login to View More
Abstract
The invention discloses a nifuratel vaginal tablet, which comprises the following components in parts by weight: 0.2-0.6 part of carbomer, 0.1-0.3 part of alginate and 1 part of nifuratel, wherein theparticle size distribution D90 of micronized nifuratel is 30-60 [mu]m. The invention also discloses a preparation method of the nifuratel vaginal tablet. According to the nifuratel vaginal tablet, the dissolution rate of the nifuratel vaginal tablet is increased by controlling nifuratel particle size distribution, the carbomer and the alginate play a role in slow release and have certain biological adhesion, so that the nifuratel vaginal tablet takes effect continuously and quickly and is not prone to falling off from the vagina, the treatment effect is guaranteed, and clothes and trousers are not polluted; and the nifuratel vaginal tablet provided by the invention is convenient to use, quick to take effect, lasting in effect and good in clinical curative effect.
Description
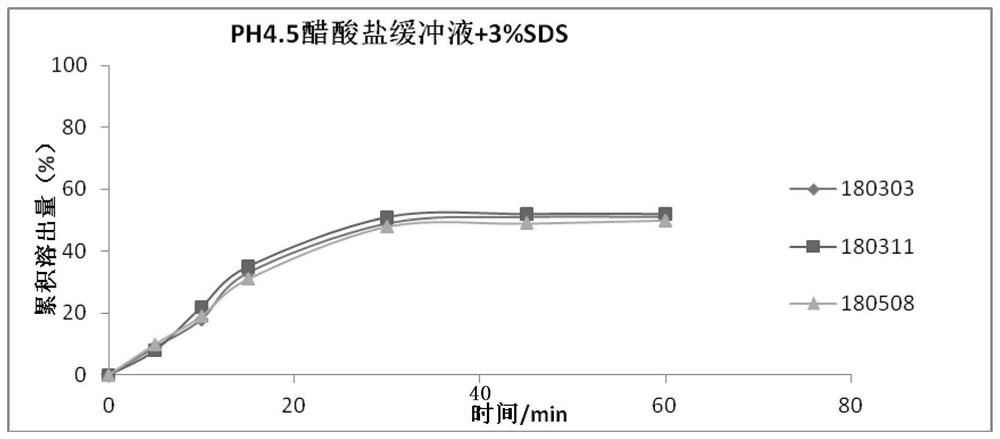
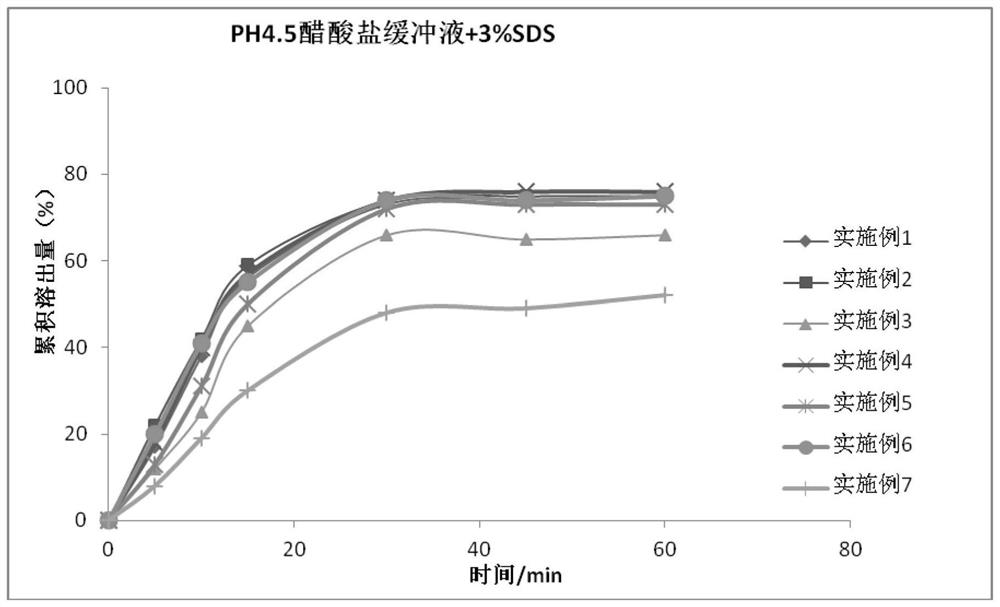
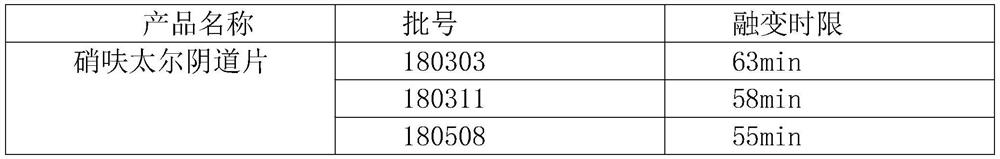
technical field [0001] The invention relates to the technical field of medicine, in particular to a nifuratel vaginal tablet and a preparation method thereof. Background technique [0002] The Chinese invention patent with the application number "201210388820.3" provides a nifuratel composition tablet with fast disintegration and high dissolution rate and its preparation method, using sodium carboxymethyl starch and cross-linked carboxymethyl cellulose Sodium is used as a disintegrant. [0003] The Chinese invention patent with the application number "201610088985.7" provides a nifuratel solid preparation and its preparation method. The tablet using the nifuratel solid dispersion has better dissolution rate and higher bioavailability. [0004] Nifuratel vaginal tablets currently on the market have low dissolution rate, slow onset of action, low bioavailability, easy to fall off from the vagina, inconvenient clinical use and poor curative effect. [0005] The melting time a...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/22A61K47/36A61K47/32A61K31/422A61P15/02
CPCA61K9/205A61K9/2027A61K9/0034A61K31/422A61P15/02
Inventor 童丰鲍勇胜
Owner BEIJING CHENG JI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com