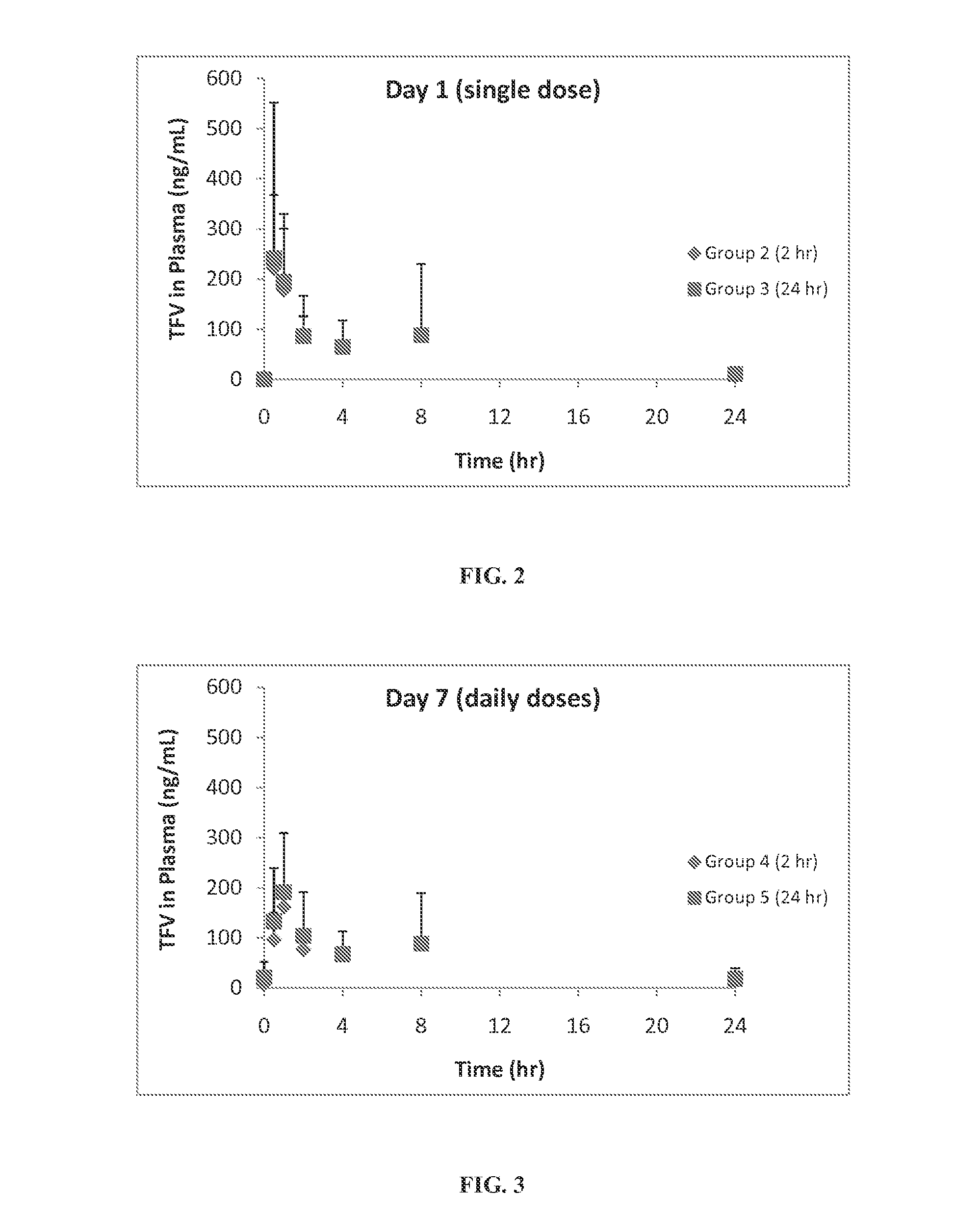
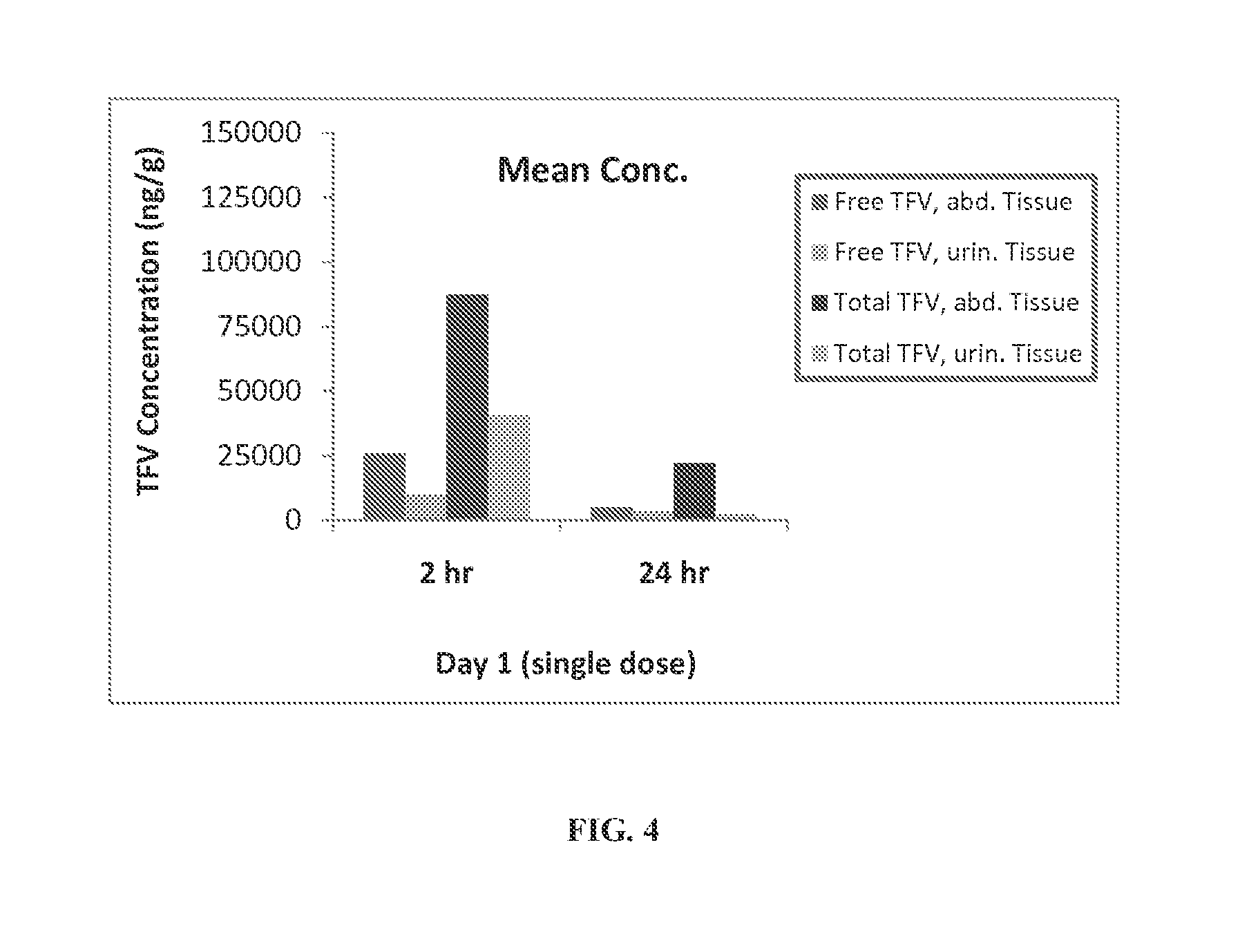
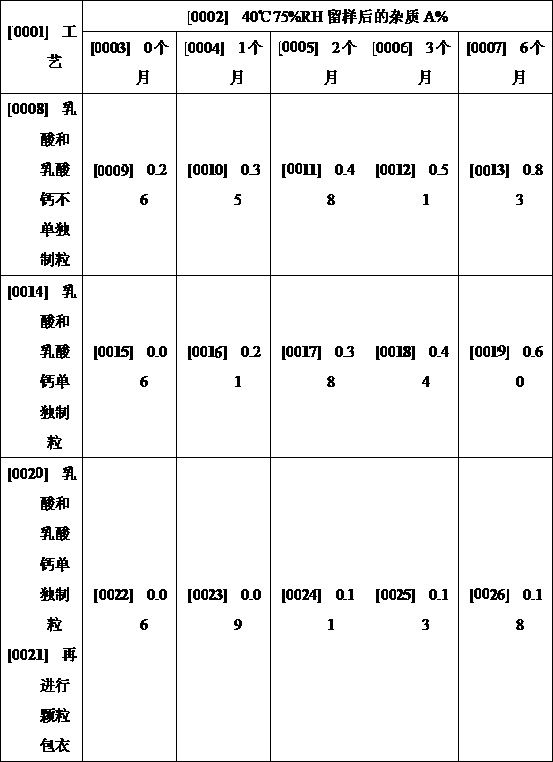
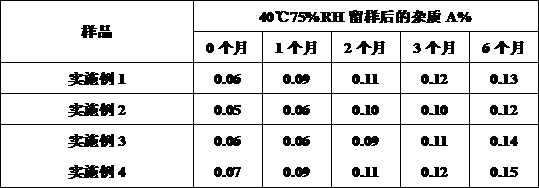
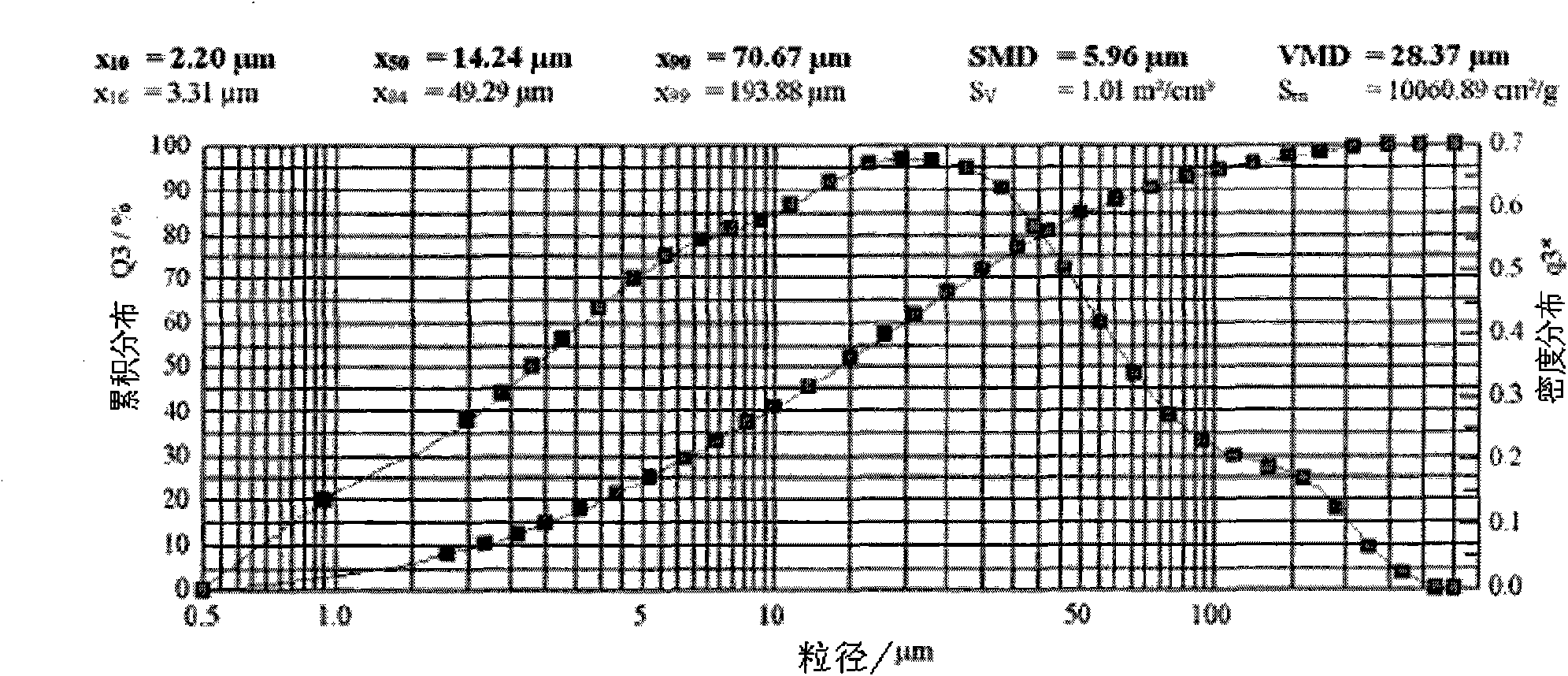
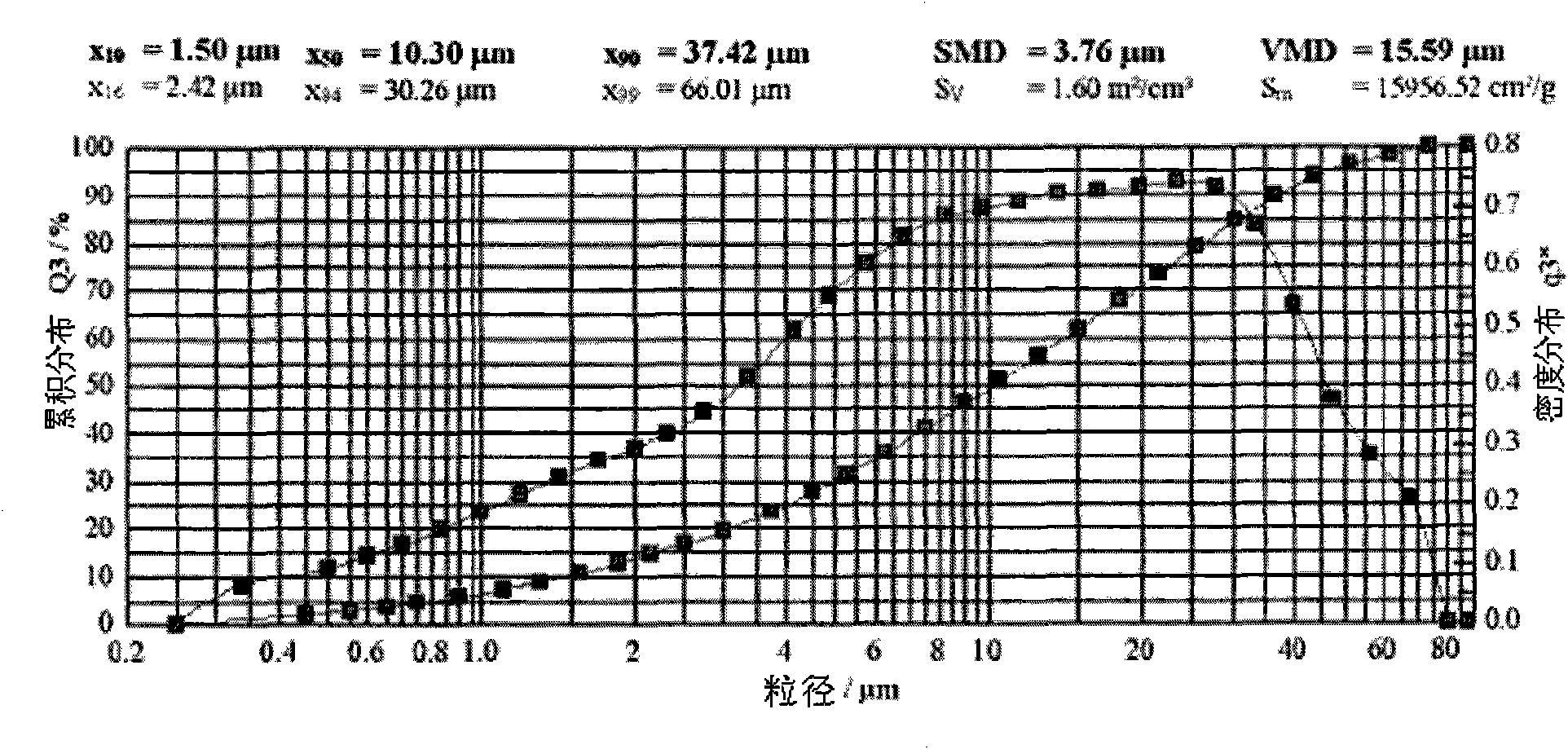
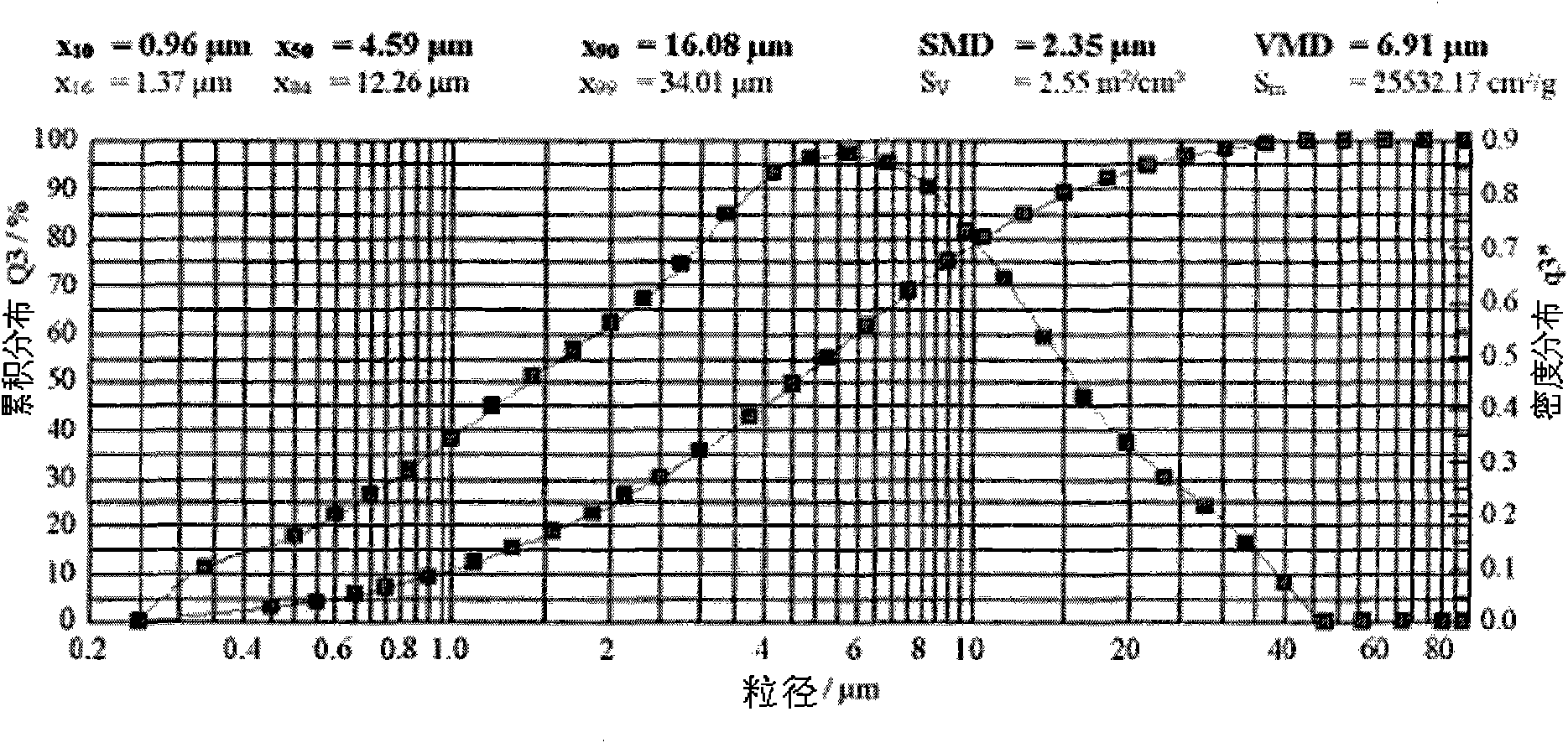
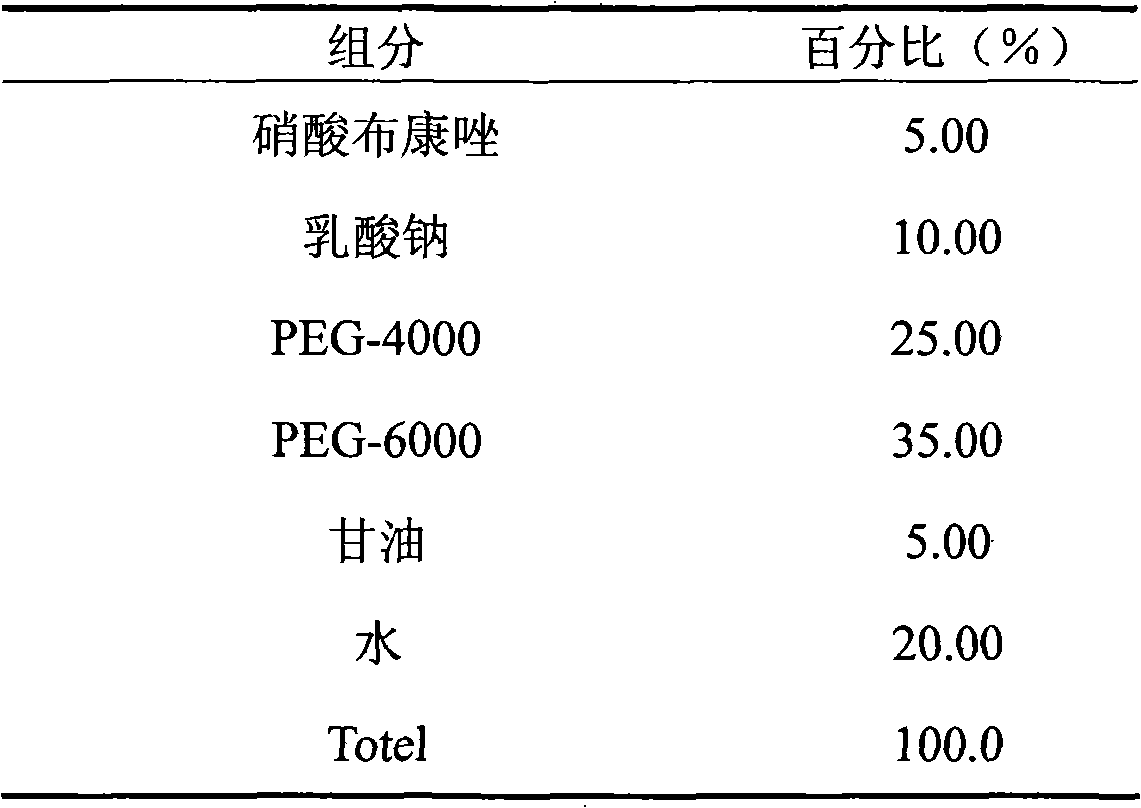
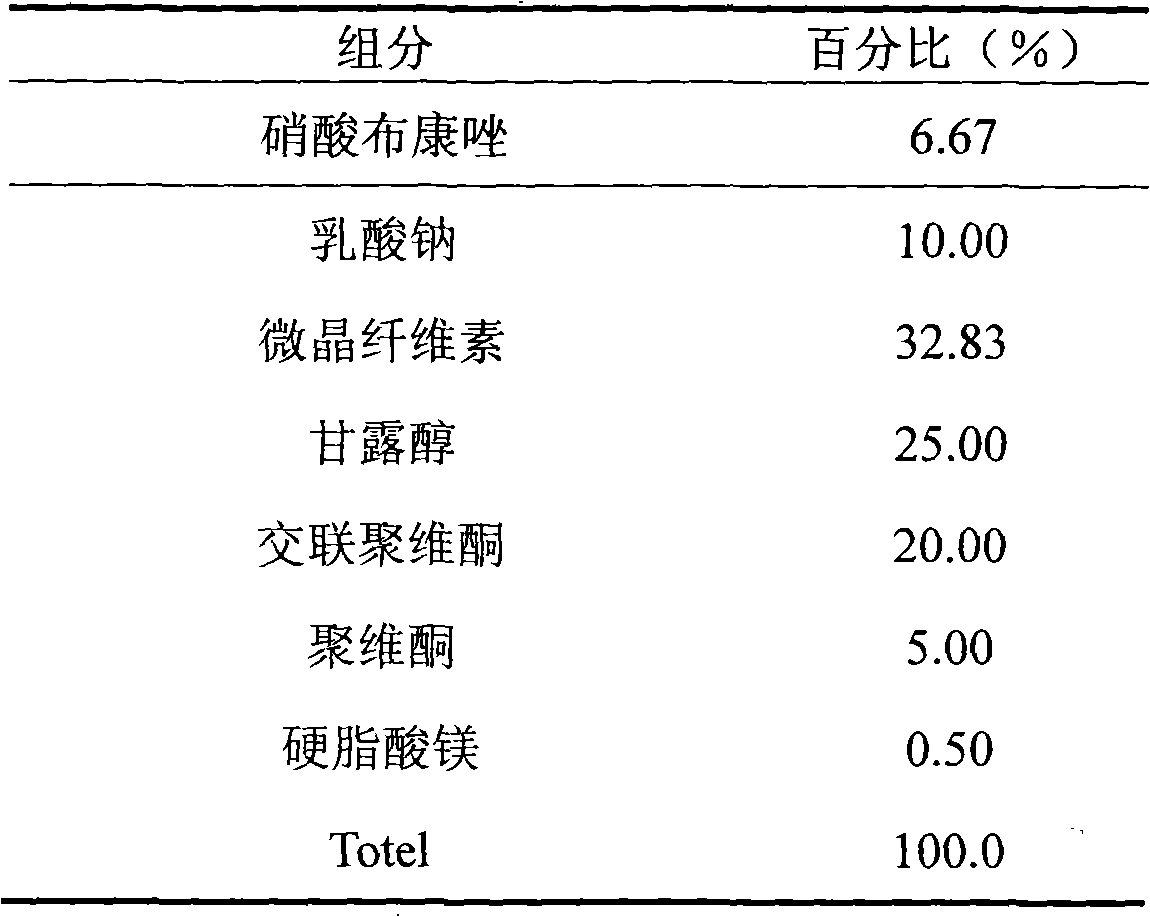
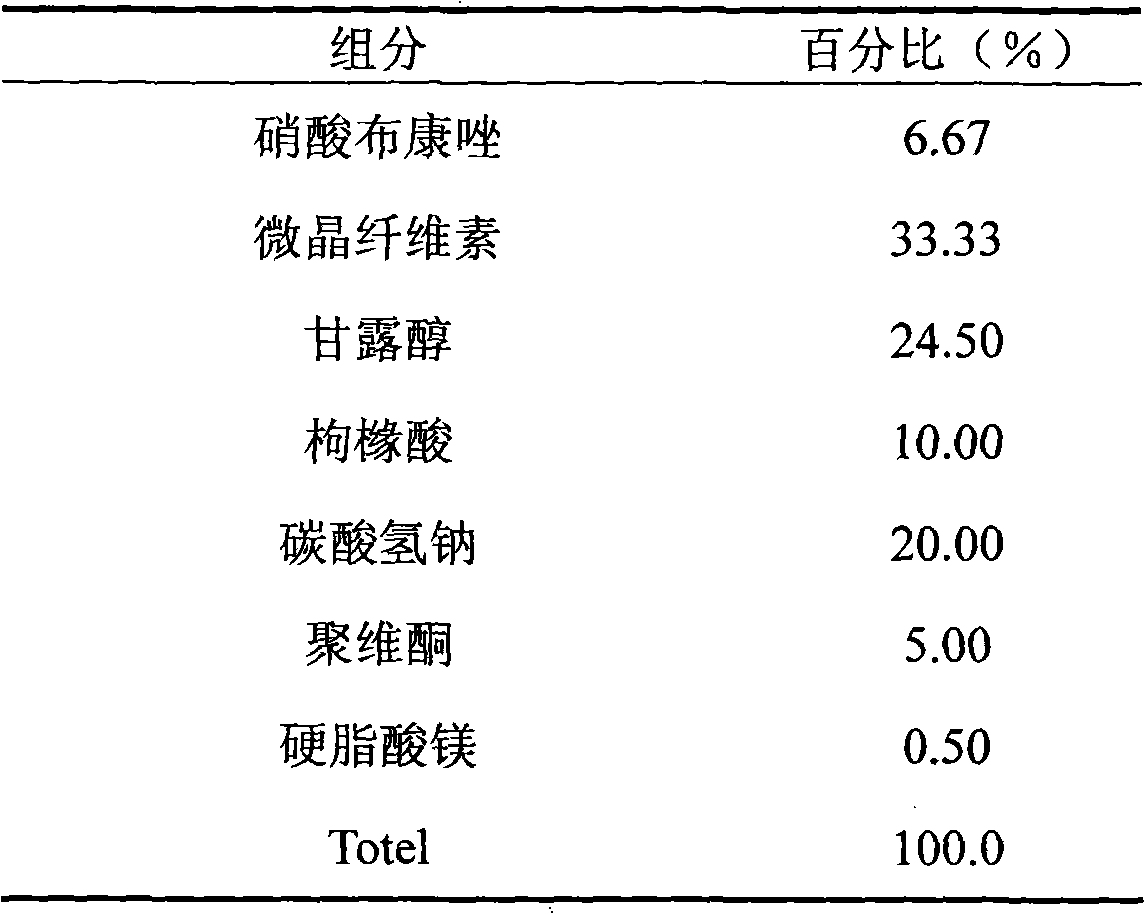
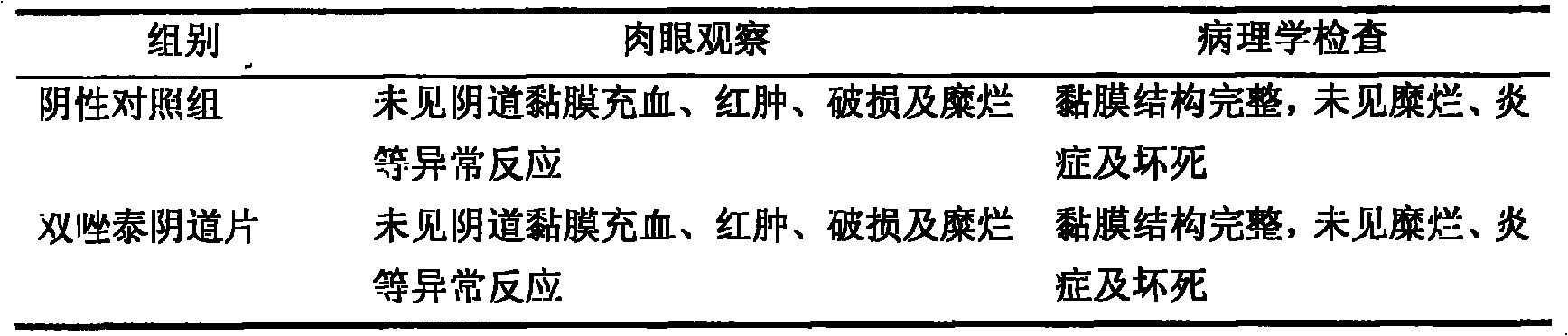
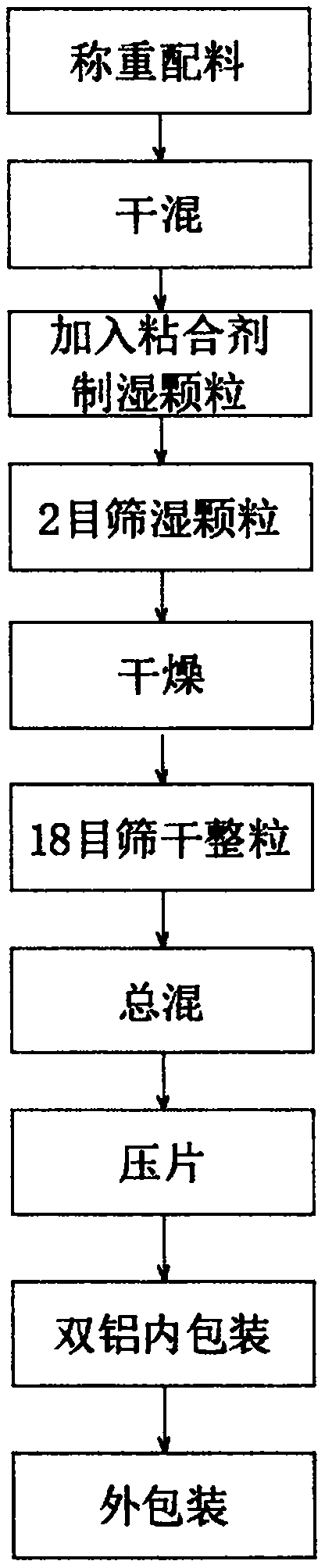
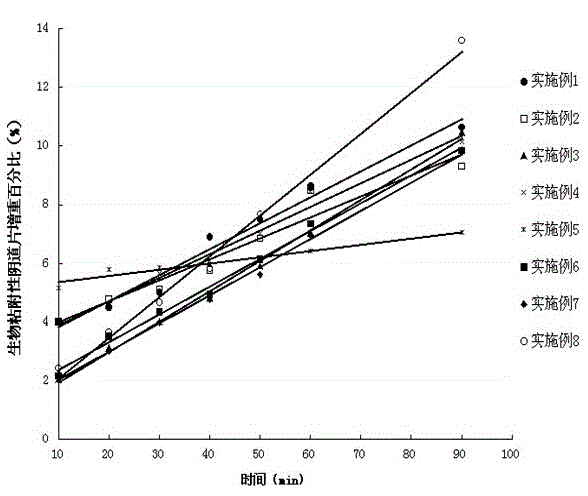
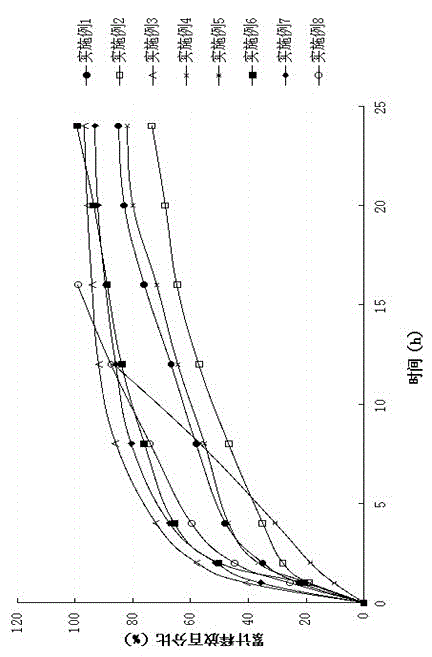
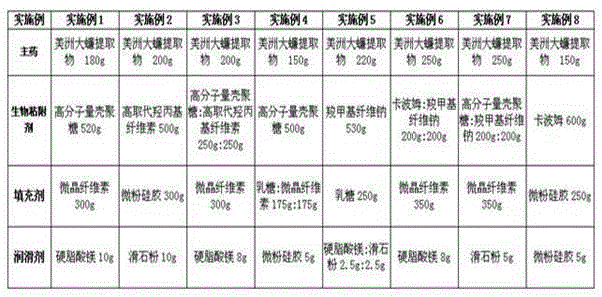
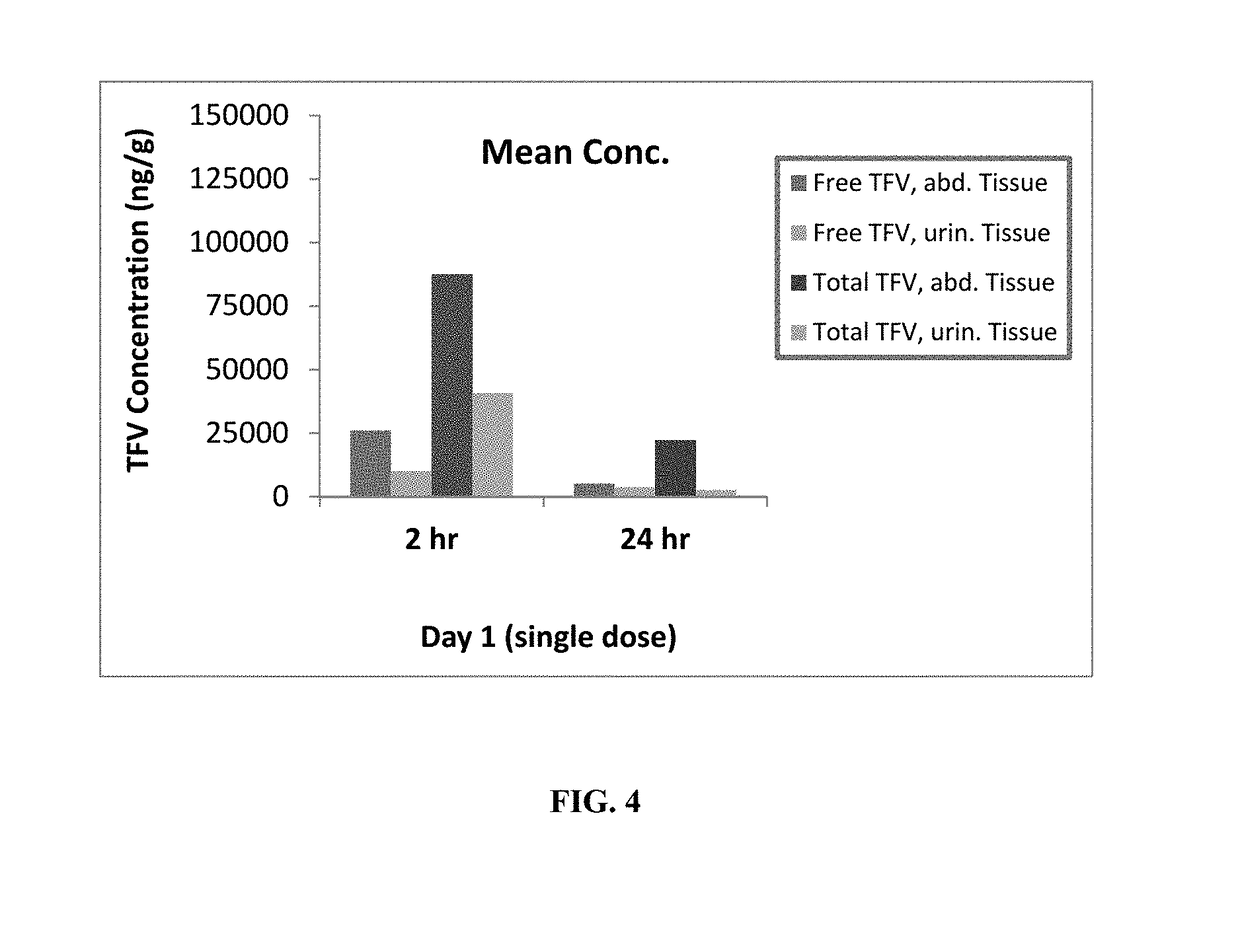
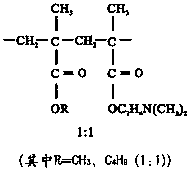Patents
Literature
43 results about "Vaginal Tablets" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CANESTEN VAGINAL TABLETS 100mg CLOTRIMAZOLE VAGINAL TABLETS 100mg (clotrimazole) Your medicine is known with either of the above names but will be referred to as Canesten throughout the following leaflet. Read all of this leaflet carefully because it contains important information for you. This medicine is available without prescription ...
Rapidly dispersible vaginal tablet that provides a bioadhesive gel
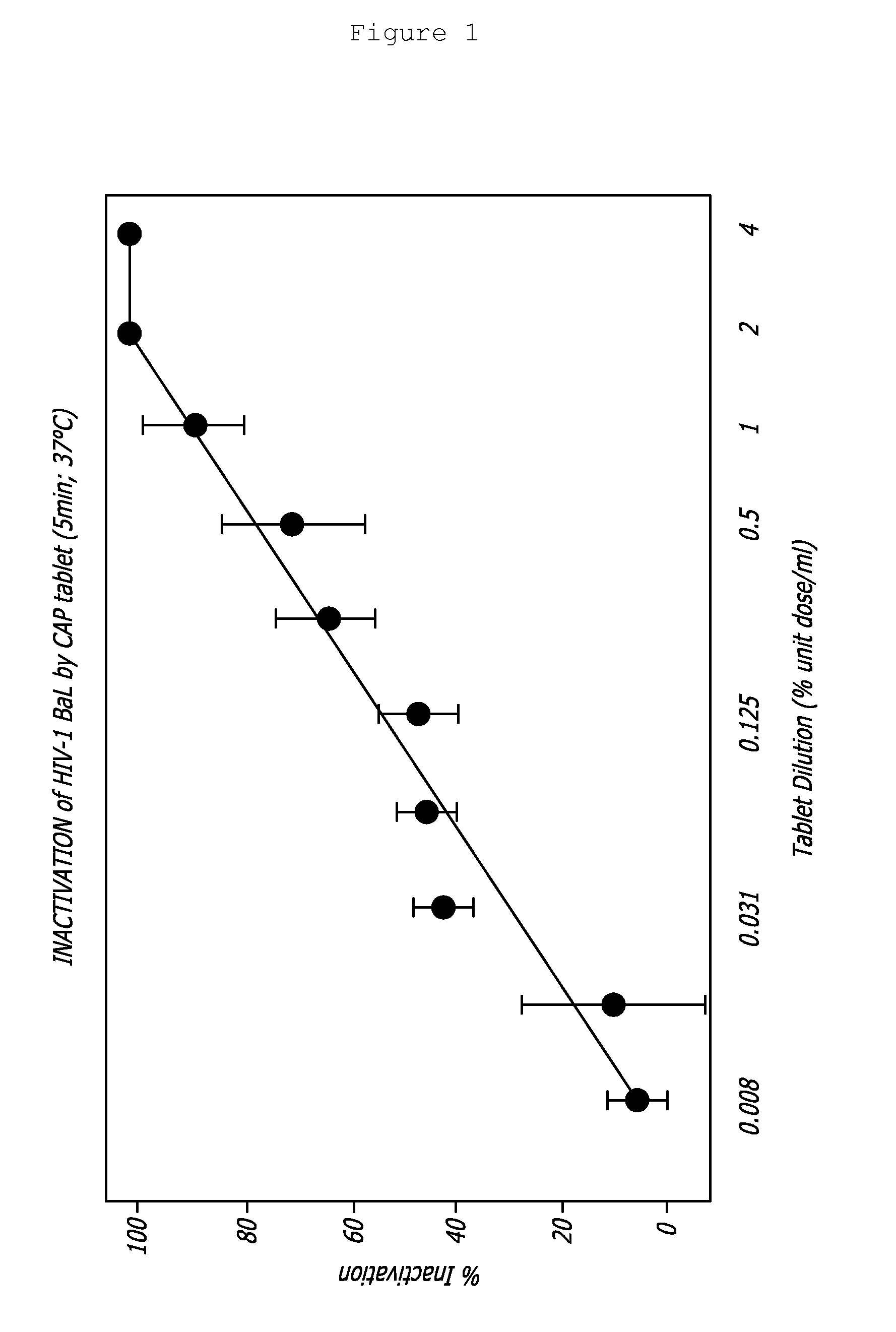
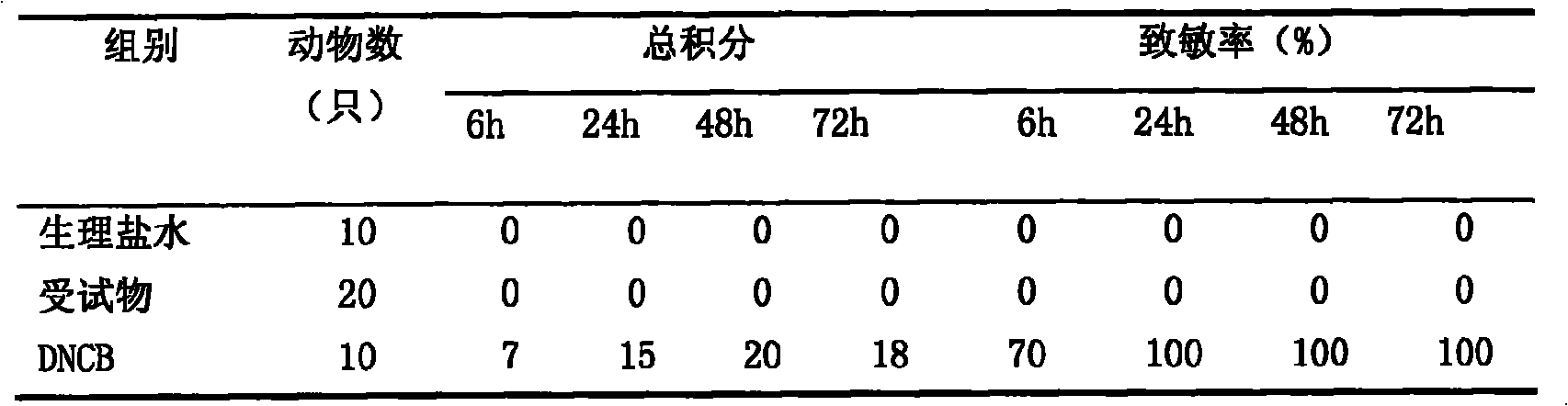
InactiveUS20110159091A1Safe and relatively inexpensive methodAvoid spreadingAntibacterial agentsBiocideBacterial vaginosisSpiroplasma
A tablet for insertion into a vagina including 0.01 to 500 mg of a vaginal medication, such as a microbicide, such as cellulose acetate 1,2-benzenedicarboxylate (CAP); 100 to 500 mg of mannitol powder; 50 to 300 mg of inert microcrystalline cellulose; 10 to 80 mg of hydroxypropyl methylcellulose; 50 to 250 mg of glycerol and optionally 2 to 4 mg of at least one preservative which protects against microbicidal contamination and discourages the growth of yeast in the vagina. The tablet which includes CAP as the vaginal medication is vaginally administered before coitus in methods for preventing the sexual transmission of HIV-1, HIV-2, herpesvirus, or an infection caused by Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Haemophilus ducreyi or Treponema pallidum. The tablet which includes CAP as the vaginal medication is vaginally administered to prevent or treat bacterial vaginosis.
Owner:NEW YORK BLOOD CENT
Compound prepn for treating women's inflammation
The present invention is compound preparation for treating womení»s inflammation, and belongs to the field of medicine technology. Each 1000 application units of the compound preparation consists of ornidazole 100-1000 g, butoconazole nitrate 10-500 g and policresulen 50-300 g. The compound preparation may be prepared into different forms, including vaginal suppository, vaginal effervescent tablet, vaginal tablet, vaginal gel, etc. The compound preparation has the functions of resisting anaerobic bacteria, resisting protoplasm, resisting mildew and resisting aerobion, and may be used in treating various womení»s infectious diseases, such as bacterial vaginitis, mycotic vaginitis, protozoal vaginitis, non-specific vaginitis, mixed infectious vaginitis, pruritus vulvae, etc.
Owner:山东特瑞林医药科技发展有限公司
HPV egg yolk antibody and application thereof in preparing drug for treating HPV infection
ActiveCN109666685AAchieve transmembrane transportSolving the problem that leaves cervical cancer with no effective solutionOrganic active ingredientsEgg immunoglobulinsPharmaceutical medicineAntibacterial activity
The invention belongs to the field of biomedicine, and particularly relates to an egg yolk antibody of HPV E6 / E7 recombined minicircle DNA and application of the antibody in preparing a drug for treating HPV infection. The egg yolk antibody is obtained by utilizing HPV E6 / E7 recombined minicircle DNA as a nuclear acid antigen for direct immunization of a laying hen. The egg yolk antibody can be used for preparing the drug for treating HPV infection. The egg yolk antibody is prepared into freeze-dried powder and subjected to superfine grinding, after lipidosome encapsulating, a nano-liposome encapsulating the egg yolk antibody is prepared, the antibody enters cells in a transmembrane mode, the high-activity anti-HPV antibody is input to a patient with positive HPV, and E6 / E7 protein in an HPV virus inside the cells is directly neutralized. The antibody is combined with antibacterial active ingredients (antibacterial compounds or gynecologic inflammation pathogen specific antibodies) andacceptable pharmaceutical carriers to be prepared into vagina spraying agents, vagina washing liquor, vagina gels, vagina soft and hard capsules, vagina suppositories, vagina membranes, vagina tablets, effervescent tablets, ointment, creams and the like, and can also be prepared into lozenges, chewable tablets, chewing gums and the like for both male and female.
Owner:江苏润洁生物科技有限公司
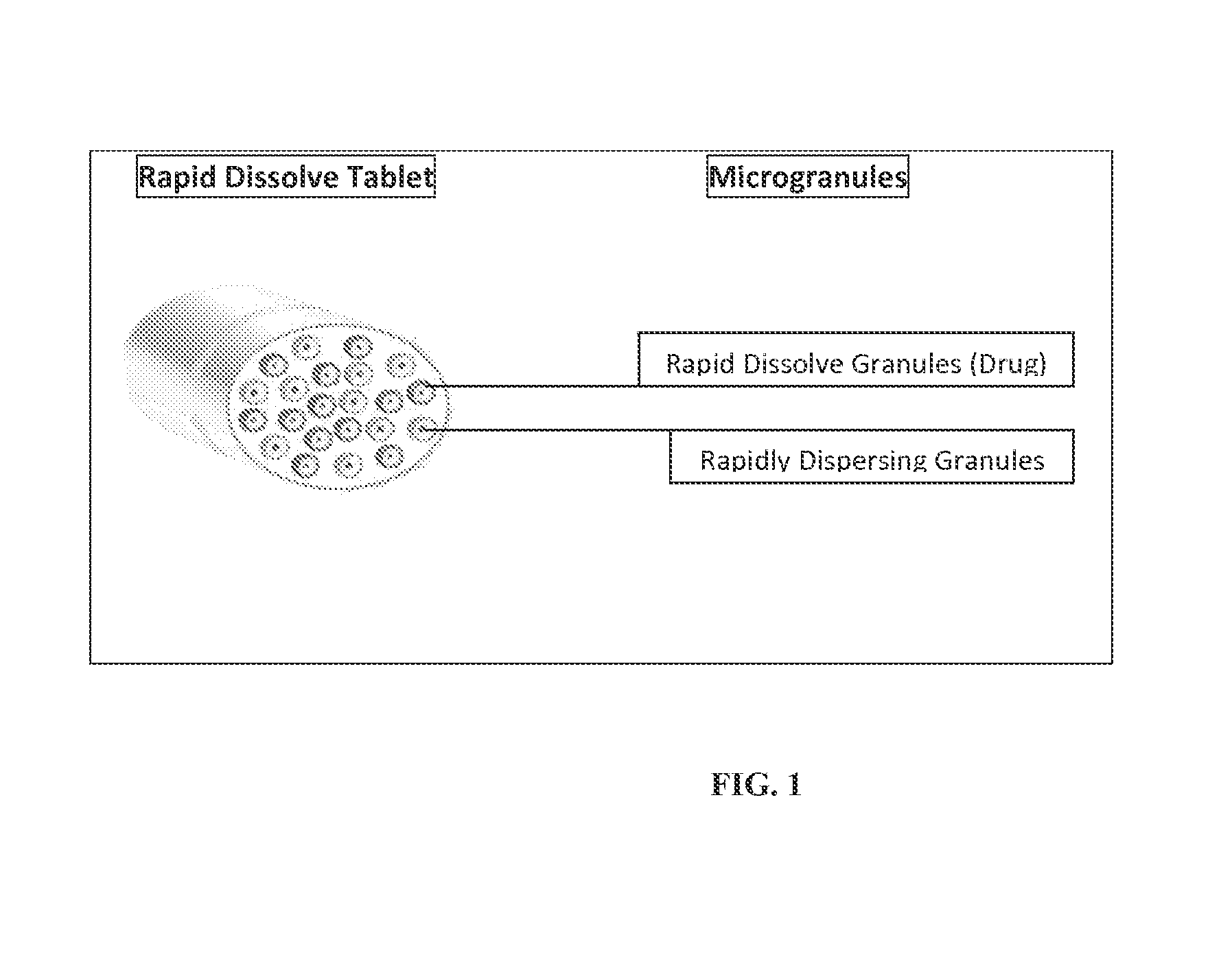
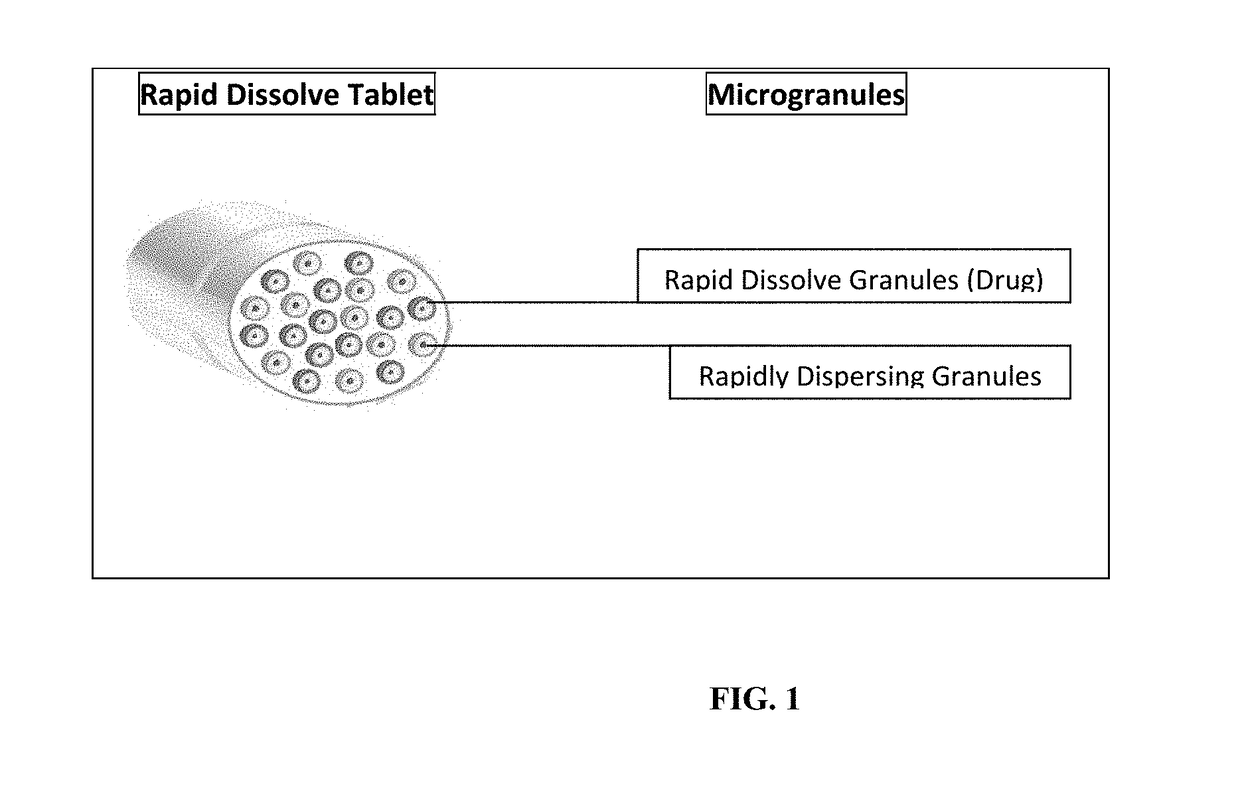
Rapid dissolve tablet compositions for vaginal administration
InactiveUS20140134246A1Enhance bioadherence of the active ingredientEnhance/sustain systemic absorptionOrganic active ingredientsBiocideSystemic absorptionPharmaceutical medicine
Disclosed herein are pharmaceutically acceptable rapid dissolve vaginal tablet compositions comprising one or more active pharmaceutical ingredients suitable for therapy via topical action or systemic absorption, and methods of making and using such compositions.
Owner:ADARE PHARM INC
Preparation method of clotrimazole vaginal tablets
ActiveCN109528673AIsolated from direct contactInhibit migrationOrganic active ingredientsAntimycoticsPrillPharmaceutical formulation
The invention discloses a preparation method of clotrimazole vaginal tablets, and belongs to the field of pharmaceutical preparations. The method comprises the following steps: 1, preparation of an adhesive solution by wet granulation; 2, preparation of a granule coating liquid; 3, preparation and coating of lactic acid and calcium lactate granules; 4, total mixing of materials; and 5, tableting and packaging. The method separately granulates lactic acid, calcium lactate and starch, and coats granules, and can effectively isolate the direct contact between raw materials and acidic components and prevent the migration of the acidic components during the tablet placing process, thereby effectively avoiding the curative effect reduction caused by the degradation of active components, and theprepared sample has good stability. The preparation process of the invention uses common equipment, can realize industrial production, and is worthy of application and promotion.
Owner:南京泽恒医药技术开发有限公司
Method for preparing policresulen vaginal tablet and quality control method
InactiveCN101234091AIncrease the effective concentrationMaintain physiological environmentPill deliverySexual disorderTherapeutic effectTrichomonas
The invention relates to a method for preparing novel gynecological policresulen vaginal tablet used for antisepsis and anti-inflammation and a quality control method thereof. The policresulen has broad-spectrum antibacterial effect on common gram-positive bacterium, gram-negative bacterium, fungus and some viruses, wherein, anaerobe trichomonas and monilia are particularly sensitive to the policresulen but vaginal lactobacilli flora is hardly effaced. The invention has the advantages that vagina local medication avoids or reduces possible untoward effect caused by systemic administration; highly effective concentration of the medicine on lesion site is in favor of developing therapeutic effect, effectively protecting physiological environment of the vagina and providing suitable life condition for good bacteria; wherein, prescription of 1,000 preparation units of the medicine is: 45-180g of the policresulen, 30-180g of filler lactose, 60-500g of starch, 50-500g of kaolin, 2-10g of bonding agent hypromellose, 6-50g of fluidizer silicon dioxide, 30-300g of disintegrating agent microcrystalline cellulose, 20-200g of polyvinyl polypyrrolidone and 1-10g of lubricant magnesium stearate.
Owner:王世锋
Gamuzhuer vagina tablet and preparation method thereof
InactiveCN102048759ATo achieve the purpose of treatmentHeavy metal active ingredientsHydroxy compound active ingredientsTraditional medicineVaginal Tablets
The invention discloses a Gamuzhuer vagina tablet and a preparation method thereof. The Gamuzhuer vagina tablet comprises the following components in percentage by weight: 5-75% of Gamuzhuer, 0-30% of biological adhesive, 0-90% of bulking agent, 0.1-10% of disintegrating agent and 0.1-10% of lubricating agent. The invention combines the biological adhesive and the Gamuzhuer, so that the tablet can be disintegrated and attached to the focal part in a shorter time, thereby achieving the purpose of treatment.
Owner:SHENZHEN JIAXUAN MEDICAL TECH DEV +1
Medicinal composition for curing gynecological diseases and preparation method thereof
InactiveCN101837067ASatisfied with the curative effectImprove securityHydroxy compound active ingredientsUrinary disorderDiseaseNon traumatic
The invention discloses a Chinese medicinal external composition for curing gynecological diseases and a preparation method thereof. The composition is prepared from amur corktree bark, hairyvein agrimonia herb and bud, common cnidium fruit, climbing groundsel herb, dried alum and borneol in a certain ratio. The composition is prepared into clinically acceptable external formulations such as suppository, external capsules, gel, ointments, vaginal tablets, vaginal effervescent tablets, vaginal films, lotion, aerosol, foaming agent and the like by a pharmaceutical conventional method. The composition has good curative effects on cervical erosion, gynecological inflammation, and urinary tract infection of women. The composition is prepared into an external preparation and is not orally administrated so as to avoid first-pass effect on liver and physiological screening effects of gastrointestinal tracts and reduce untoward effect. The external preparation can directly act on afflicted parts, and has the advantages of strong pertinence, general actions through skin and mucosa, quick medicinal absorption, less interference to internal environment of body, non-traumatic treatment mode, simple operation, good safety of clinical use, high cure rate, base strengthening of Chinese medicaments, and less side effect. The composition can effectively eliminate cause of diseases, can safely and effectively cure diseases under guidance of doctors and is easy to popularize.
Owner:北京康正元科技发展有限公司武汉分公司
Clotrimazole vaginal tablets
InactiveCN106420726AFine grainGood sustained releaseOrganic active ingredientsAntimycoticsPrillYeast vaginitis
The invention discloses clotrimazole vaginal tablets and a production method thereof. Raw materials and process parameters are optimized comprehensively, and it is unexpectedly found that the clotrimazole vaginal tablets have a good slow release effect, are not required to be combined with oral medication and have a good control effect on recurrence of recurrent vulvovaginal candidiasis under the optimized parameters. The method comprises steps as follows: (1) material preparation in parts by weight: (2) pretreatment: all raw and auxiliary materials, except kushenin powder and nano tourmaline powder, are sieved by an 80-mesh sieve for later use; the kushenin powder is sieved by a 200-mesh powder for later use; the particle size of the nano tourmaline powder is selected to range between 8 nm and 12 nm; (3) granulation: the raw and auxiliary materials are weighed according to a prescription, placed in a high-speed mixing granulator for mixing and subjected to wet granulation with a lactic acid aqueous solution; (4) drying: prepared wet granules are dried at 60 DEG C for 4 h; (5) granulation and whole mixing: dried granules are placed in a 3D motion mixer to be uniformly mixed as a batch; (6) tableting: tableting can be performed after the granules pass inspection, and each table contains 500 mg of clotrimazole.
Owner:ZHEJIANG SHENGBOKANG PHARMA CO LTD
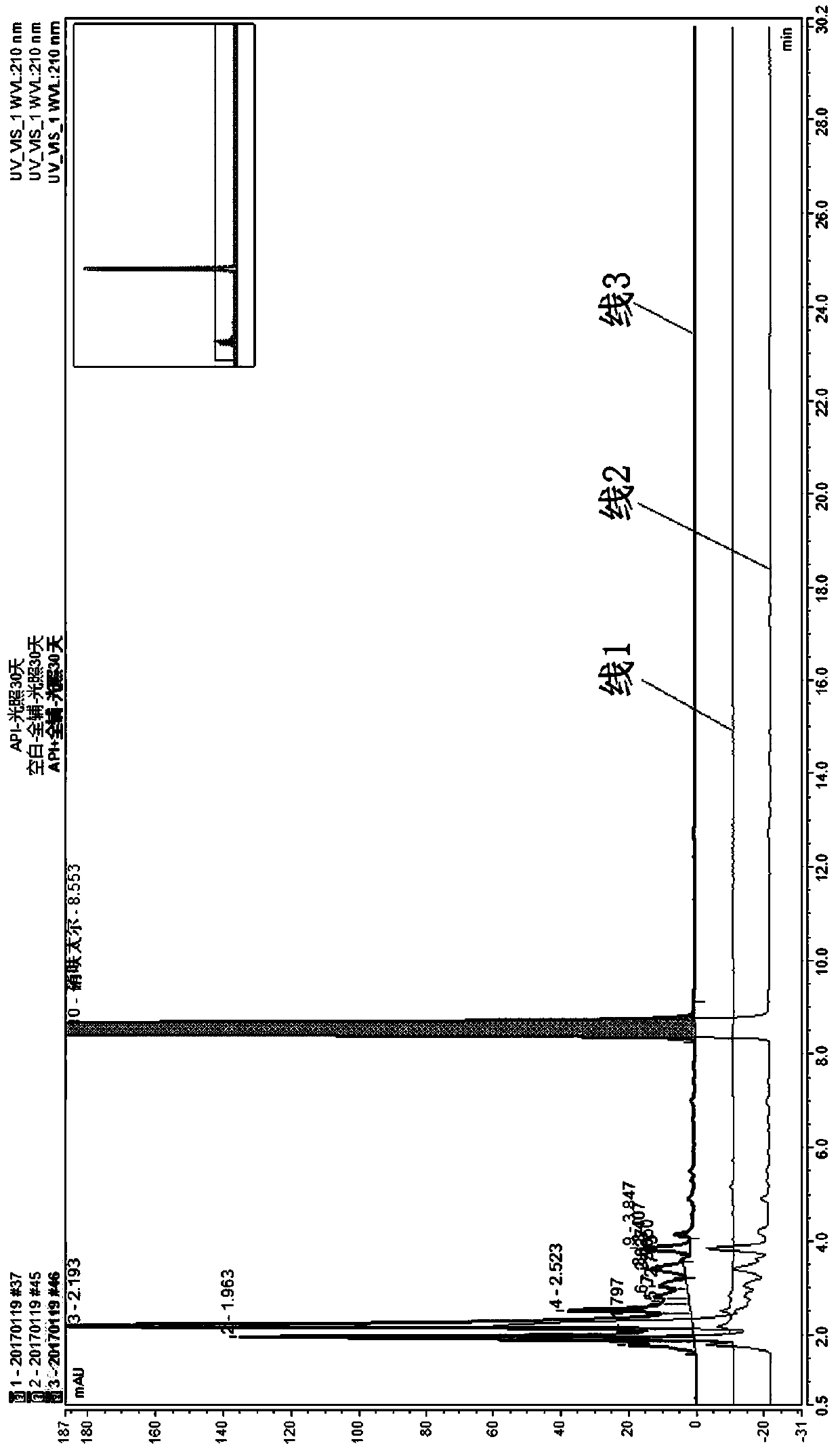
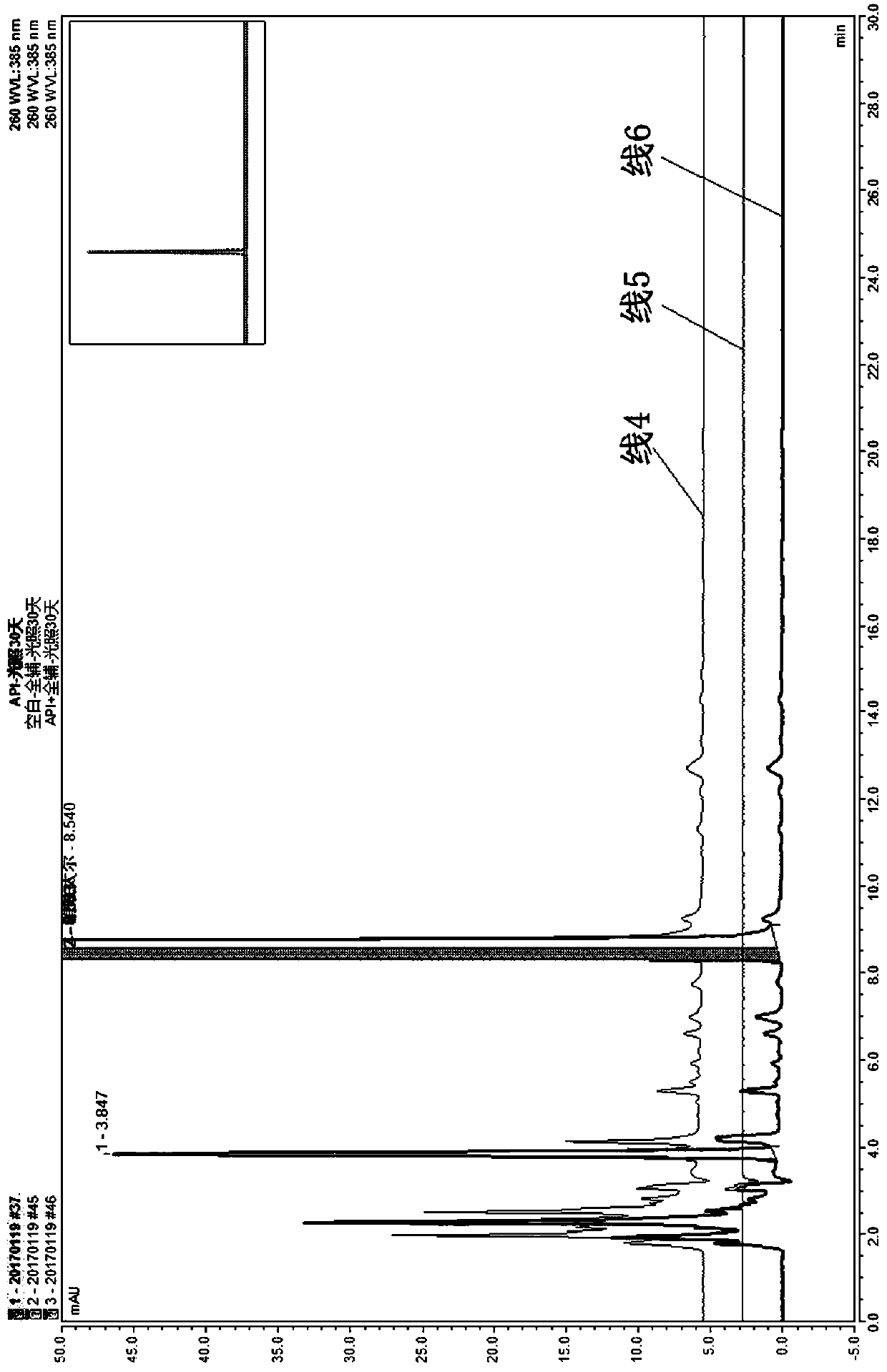
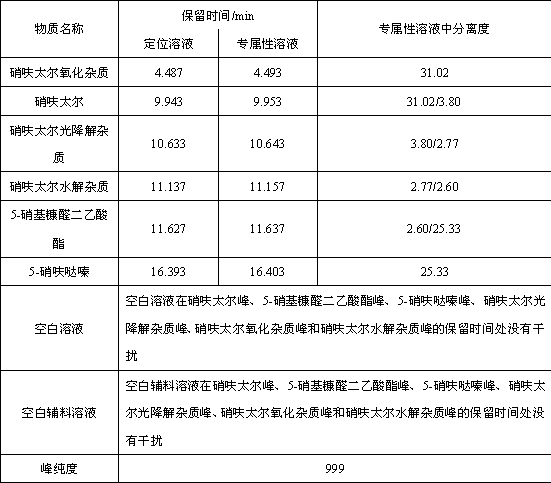
Method for controlling impurities of nifuratel vaginal tablet
InactiveCN109839456AResolve interferenceSolve the separation problemComponent separationPotassium hydroxideGradient elution
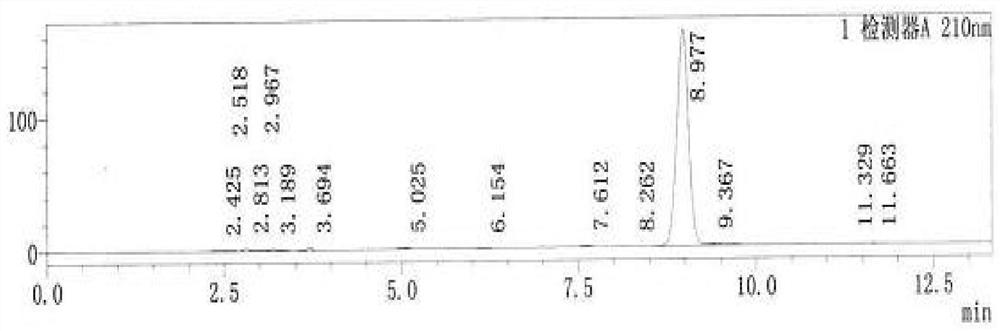
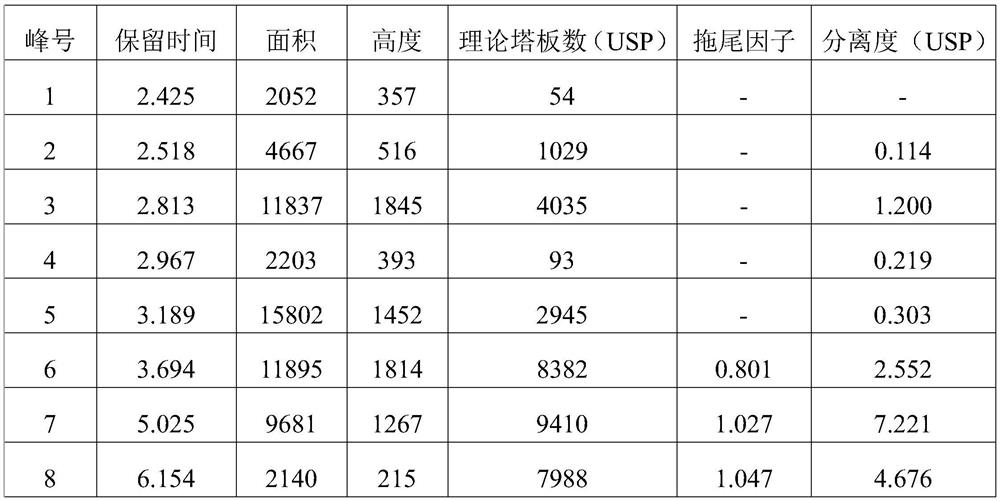
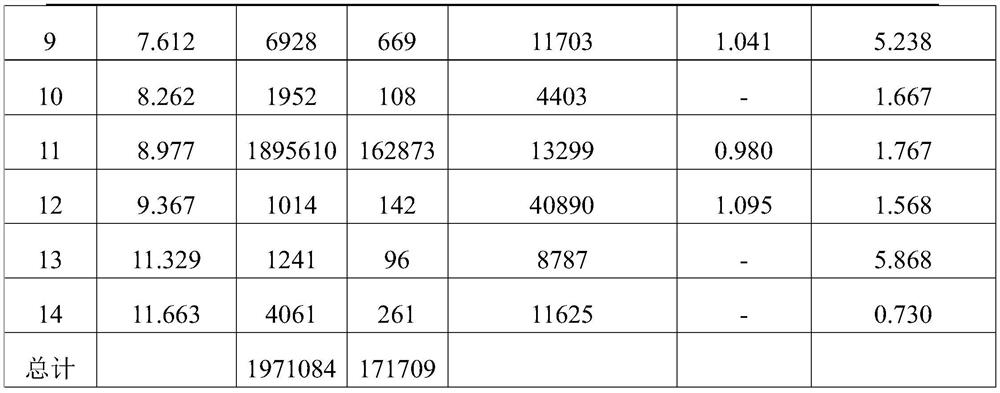
The invention relates to the technical field of drug analysis, and in particular relates to a method for controlling impurities of a nifuratel vaginal tablet. The method is a gradient elution method with a detection wavelength of 260 nm. The mobile phase is phase A: a 9 to 11 mM KH2PO4 solution, and a potassium hydroxide aqueous solution is used to adjust pH. The volume ratio of phase B: acetonitrile: methanol is 18:82 to 22:78. The method provided by the invention can solve the problems of excipient interference and impurity separation at the same time, and can comprehensively and effectivelydetect the content of each impurity. An effective method is provided for impurity control of nifuratel in preparation. The method provides a basis for the formulation of preparation quality standards.
Owner:ANHUI PIOM PHARMA
Vagina tablet for treating cervicitis, cervical erosion, and preparation method
InactiveCN1958007ALong retention timeOvercome deficienciesHydroxy compound active ingredientsPill deliveryCervicitisCervix
A vaginal tablet for treating cervicitis and cervical erosion is prepared from phellodendron bark, flavescent sophora root, catechu, baked alum and borneol. Its preparing process is also disclosed.
Owner:哈尔滨儿童制药厂有限公司
Danazol vaginal tablet process and quality control method
The invention relates to a preparation method and a quality control method of a new drug, Danazol Vaginal Tablets, for treating endometriosis with clear dysmenorrhea symptom but light physical sign. The drug can be delivered via cavitary mucosa, so as to prevent or relieve the possible adverse reactions due to systemic drug delivery; the effective drug concentration at lesion site is high, so as to be in favor of exert curative action; and the added lactic acid-calcium lactate buffer system results in the drug with pH of 3.5-4.0 that is similar to the physiological pH in vagina, so as to effectively retain physiological environment in vagina, provide existing conditions suitable for vaginal beneficial bacteria, and promote rehabilitation.
Owner:王世锋
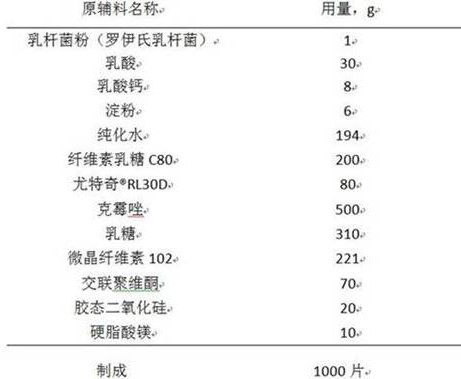
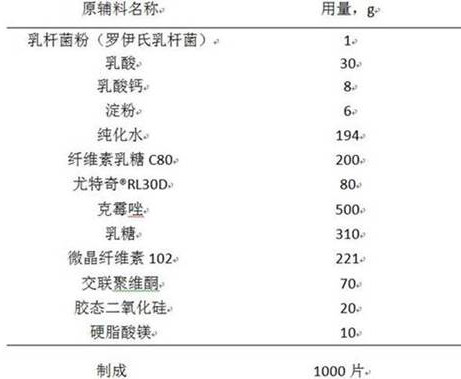
Clotrimazole vaginal tablet composition and preparation method thereof
ActiveCN111789838AImprove complianceGood antibacterial effectAntimycoticsUnknown materialsBiotechnologyCALCIUM LACTOBIONATE
The invention relates to a clotrimazole live lactobacillus composition and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The clotrimazole live lactobacillus vaginal tablet disclosed by the invention comprises 500mg of clotrimazole; 1mg of lactobacillus powder; 20mg to 30mg of lactic acid; 8-12mg of calcium lactate; 6-12mg of starch; 200-300mg of cellulose lactose C80; 66mg to 180mg of eudragit-RL30D; 225mg to 314mg of lactose; 170mg to 225mg of microcrystalline cellulose; 70mg of crospovidone; 20mg of colloidal silicon dioxide; and 10 mg of magnesium stearate. The invention provides the clotrimazole live lactobacillus vaginal tablet.
Owner:DISHA PHARMA GRP
Antibacterial gynecology externally used pharmaceutical combination
ActiveCN101269067AImprove stabilityImprove antibacterial propertiesOrganic active ingredientsAntisepticsPharmacyAdditive ingredient
The invention discloses an external gynecological drug compound which consists of an active ingredient butoconazole nitrate and other drug carriers acceptable in pharmacy. The external gynecological drug compound has the forms of suppositories, vaginal tablets and vaginal effervescent tablets and can be used for treating the fungal infection outside the vagina caused by mycotoruloides.
Owner:万全万特制药(厦门)有限公司
Vaginal tablet with clindamycin and metronidazole
The invention relates to a vaginal tablet of clindamycinum and metronidazole, which uses the salts or esters of clindamycinum and metronidazole as the active constituents, and the conventional tablets as the auxiliary material.
Owner:程月发
Vagina administration medicine for treating and preventing woman vagina inflammation and preparation method thereof
InactiveCN100998816ABeautiful appearanceImprove bioavailabilityAntibacterial agentsHydroxy compound active ingredientsDiseasePathogenic microorganism
A Chinese medicine in the form of soft-capsule, tablet, or capsule for preventing and treating vaginitis, pudendal itching, morbid leukorrhea, etc and preventing venereal disease is prepared from 7 Chinese-medicinal materials including rhubarb, hineysuckle flower, alum, borneol, etc. Its preparing process is also disclosed.
Owner:RENHE GRP DEV CO LTD
Metronidazole vaginal tablet process and quality control method
InactiveCN101283989AEasy to useUse cleanOrganic active ingredientsPill deliveryDiseaseChlorhexidine Acetate
The invention relates to a preparation method and a quality control method of a new gynecologic drug, Metronidazole, Clotrimazole and Chlorhexidine Acetate Vaginal Tablets, with antibacterial and anti-inflammatory effects. The added lactic acid-calcium lactate buffer system results in a drug with pH of 3.5-4.0 t that is similar to the physiological pH in vagina, so as to effectively retain physiological environment in vagina, provide existing conditions suitable for vaginal beneficial bacteria, and promote rehabilitation. The main preparation method comprises the steps of compounding, granulating, grading, mixing, and tabletting. The quality control method comprises the steps of collecting one dosage unit of the product, grinding, adding 100mL water, shaking for 10min, and testing pH (should be 3.5-4.0); testing melting time limit (should be 30min); and measuring the nominal content of the three components by high performance liquid chromatography (should be both in the range of 90.0-110.0%).
Owner:何文健
Chinese medicinal composition for external use and preparation method thereof
InactiveCN101879237ASatisfied with the curative effectImprove securityHydroxy compound active ingredientsUrinary disorderDiseaseGynecologic Diseases
The invention discloses a Chinese medicinal composition for external use and a preparation method and application thereof. The composition consists of amur corktree bark, herba agrimoniae, common cnidium fruit, climbing groundsel herb, dried alum and borneol according to certain proportion; and according to a pharmaceutical conventional method, the composition is prepared into clinical acceptable formulae for external use, such as suppositories, capsules for external use, gelata, ointments, vaginal tablets, vaginal effervescent tablets, vaginal pellicles, washing liquor, aerosols, forming agents and the like. The composition has a better therapeutic effect of treating gynecologic diseases, such as cervical erosion, gynecologic inflammations and urinary tract infection; the composition is prepared into an external preparation and is not orally taken to avoid a liver first-pass effect and a gastrointestinal tract physiological checkpoint effect and reduce adverse reactions; the external preparation can directly act on sick parts, has strong pertinence, can act on a whole body through skins or mucous membranes, can be absorbed quickly, has small interference on the internal environment of an organism, is a non-invasive treatment mode, is simple and convenient to operate, has high safety and high curative ratio in clinical application, is a Chinese medicament for enhancing the body resistance, has small side effects, can effectively and thoroughly eradicate causes of diseases, can be used for performing safe and effective treatments under the guidance of doctors, and is favorable for popularization.
Owner:北京康正元科技发展有限公司武汉分公司
Dequalinium vaginal tablets and preparation method thereof
InactiveCN105919959AReasonable compositionUnique craftAntibacterial agentsOrganic active ingredientsLACTOSE MONOHYDRATELactose
The invention discloses dequalinium vaginal tablets and a preparation method thereof. Each dequalinium vaginal tablet comprises, by weight, 10mg of dequalinium, 50-100mg of lactose monohydrate, 25-50mg of microcrystalline cellulose and 1-3mg of magnesium stearate. The preparation method which uses the production equipment of common tablets is simple in process and low in cost. The dequalinium vaginal tablets have the advantages that the tablets are fast in disintegration and quick in action, patients accept the tablets with pleasure, and the like.
Owner:ANHUI YIXINMING PHARMA TECH
Method for determining content of clindamycin phosphate vaginal tablets
ActiveCN112684056AEasy to separateHigh measurement accuracyComponent separationFluid phaseClindamycin Phosphate
The invention provides a method for determining the content of clindamycin phosphate vaginal tablets. The method adopts high performance liquid chromatography for detection, selects mobile phase components, adopts scientific proportioning, adopts gradient elution and sets elution conditions, and can rapidly and accurately detect the content of clindamycin hydrochloride for injection so as to achieve the purpose of simply, conveniently, rapidly and accurately controlling the product quality.
Owner:HAINAN HAISHEN TONGZHOU PHARM CO LTD
Production technology of miconazole nitrate vaginal tablets
InactiveCN110237042AIncrease profitImprove production efficiencyOrganic active ingredientsAntimycoticsPharmacyDry mixing
The invention provides a production technology of miconazole nitrate vaginal tablets, and relates to the technical field of a medicine making technology. The production technology of miconazole nitrate vaginal tablets comprises the following steps of performing weighing and blending, performing dry mixing to obtain wet granules, performing drying, performing granule trimming, performing total mixing, performing tabletting, performing packaging, and performing warehousing: performing packaging, performing total inspection, and performing warehousing. The miconazole nitrate vaginal tablets are produced through an assembly line of whole automated equipment, so that the making efficiency of the whole products can be improved, the yield of unit time can be increased, enterprise profits are increased, quality control is performed for products produced through multiple working procedures, the quality of products produced through a single working procedure can be guaranteed, control on the quality of finished products is realized, the content of defectives in the pharmacy process is reduced, and the whole rate of finished products in the whole pharmacy technological process is increased.
Owner:JINAN LIMIN PHARMA
Method for preparing metronidazole furanzolidon vaginal tablet and quality control method
InactiveCN101234092AIncrease the effective concentrationPromote recoveryOrganic active ingredientsPill deliveryBacterial vaginosisWhole body
The invention relates to a method for preparing novel gynecological metronidazole furazolidone vaginal tablet used for antisepsis and anti-inflammation and a quality control method thereof. The medicine is used for curing cervicitis, cervical erosion, trichomonas vaginitis, bacterial vaginosis and colpitis mycotica. The invention has the advantages that vagina local medication avoids or reduces possible untoward effect caused by systemic administration; highly effective concentration of the medicine on lesion site is in favor of developing therapeutic effect. Prescription of 1,000 preparation units of the medicine is: 3-26g of metronidazole, 3-26g of furazolidone, 40-395g of alumen, 0.6-6g of borneol, 6-55g of frankincense, 40,003-30g of polyethylene glycol, 40-400g of lactose, 50-500g of starch, 1-30g of hypromellose, 10-100g of silicon dioxide, 30-300g of microcrystalline cellulose, 30-300g of polyvinyl polypyrrolidone (additionally provided 10-100g) and 1-20g of magnesium stearate.
Owner:屠瑞丽
Biological adhesive vaginal tablet of periplaneta americana extract and preparation method of biological adhesive vaginal tablet
ActiveCN104434992AGood bioadhesionPromote absorptionOrganic active ingredientsAnthropod material medical ingredientsEscherichia coliMonilinia laxa
The invention belongs to the technical field of pharmacy, and particularly relates to a biological adhesive vaginal tablet of a periplaneta americana extract and a preparation method of the biological adhesive vaginal tablet. The biological adhesive vaginal tablet is characterized by being prepared from the following raw materials in parts by weight: 15-25 parts of periplaneta americana extract, 40-60 parts of a biological adhesive, 25-35 parts of a filler and 0.1-5 parts of a lubricating agent. Chitosan in the biological adhesive vaginal tablet is a natural amino polysaccharide from marine organisms, and has an inhibiting effect on experimental strains such as staphylococcus epidermidis, staphylococcus aureus, escherichia coli, pseudomonas aeruginosa and monilia albicans. The chitosan not only has excellent biological adhesion performance, but also is capable of opening tight connection of epithelial cells, promoting medicine absorption, and promoting wound healing and repairing, and cooperatively acts on the wounds together with a kangfuxin lotion.
Owner:KUNMING SINOWAY NATURAL PHARMA
Ketoconazole vaginal tablet and preparation method thereof
InactiveCN101574321AExtended retention timeIncrease concentrationOrganic active ingredientsAntimycoticsSide effectMagnesium stearate
The invention relates to a ketoconazole vaginal tablet and a preparation method thereof. The prescription of the vaginal tablet comprises the following components according to the parts by weight: 1 of ketoconazole, 0.01-0.2 of carboxyrnethyl starch sodium, 0.01-0.2 of sodium bisulfite, 0.1-2 of lactose; 0.001-0.02 of polysorbate 80, 0.001-0.2 of polyvidone K30; 0.001-0.1 of magnesium stearate and 0.001-0.1 of talcum powder. Compared with the prior art, the ketoconazole vaginal tablet not only overcomes the defects of easy loss and clothing pollution of pessary, but also avoids the defects of demanding production conditions, inconvenient storage, and the like which are caused by a vaginal effervescent tablet using special accessories. Meanwhile, the ketoconazole vaginal tablet improves the cure effect in using ketoconazole to cure colpitis, reduces toxic and side effects, provides more choices for clinical doctors and patients and is one of ideal ketoconazole partial-vagina administration medicament forms.
Owner:SHANGHAI PUKANG PHARMA
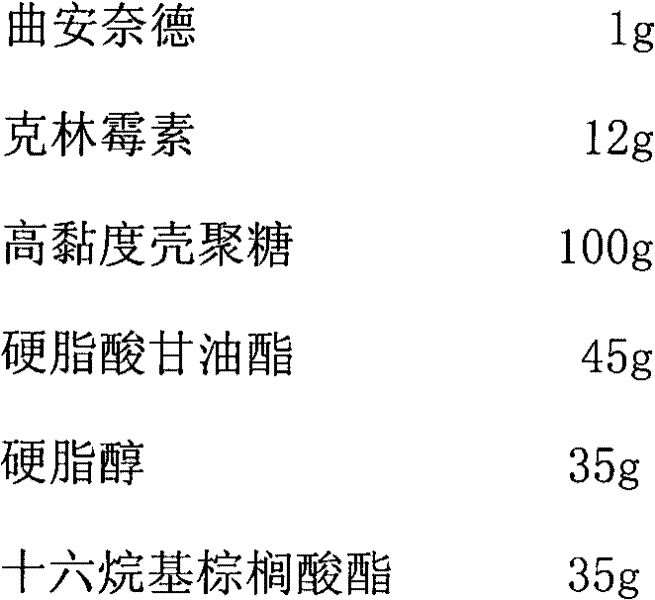
Method for treating cervical erosion by using preparation containing triamcinolone acetonide
InactiveCN102416018APrevent recurrenceAnti-inflammatoryOrganic active ingredientsAntipyreticEffective actionAntibiotic Y
The invention provides a method of applying a topical preparation containing triamcinolone acetonide in treating or preventing cervical erosion. The preparation can comprise triamcinolone acetonide and other accessories or be a composition of triamcinolone acetonide and one antibiotic or more antibiotics. According to the treatment method provided in the invention, a variety of topical dosage forms of the preparation containing triamcinolone acetonide can be used, e.g., vaginal cream, vaginal gelata, vaginal suppository, vaginal effervescent tablets, vaginal tablets, vaginal ointment, etc. The preparation used in the method has anti-inflammatory and vasoconstrictive effects, has effective action on reducing, shallowing and eliminating an erosion area and is effective in inhibiting recurrence of triamcinolone acetonide.
Owner:JIAXING FUTEJI BIOLOGICAL TECH
Chinese medicinal composition for treating endometriosis
InactiveCN102847071AImprove symptoms of dystopiaInhibit and eliminate growthUnknown materialsSexual disorderSalvia miltiorrhizaSuppository
The invention provides a Chinese medicinal composition for treating endometriosis. The composition is composed of sliced cornu cervi, Salvia Miltiorrhiza, radix paeoniae rubra, Chinese angelica, Rhizoma sparganii, zedoary, Prunella vulgaris, thunberg fritillary bulb and eucommia. The Chinese medicinal composition for treating endometriosis can be in the forms of granules, tablets, capsules, oral liquids, suppositories or vaginal tablets. With a reasonable prescription, the Chinese medicinal composition provided in the invention cares both symptoms and root causes, treats renal deficiency and blood stasis concurrently, has a clinical effective rate up to 90.13%, and can significantly improve the symptoms of endometriosis, inhibit and eliminate endometrium growth.
Owner:WUJIANG SHI FINE WORKMANSHIP & ALUMINUM WORD MANUFACTORY
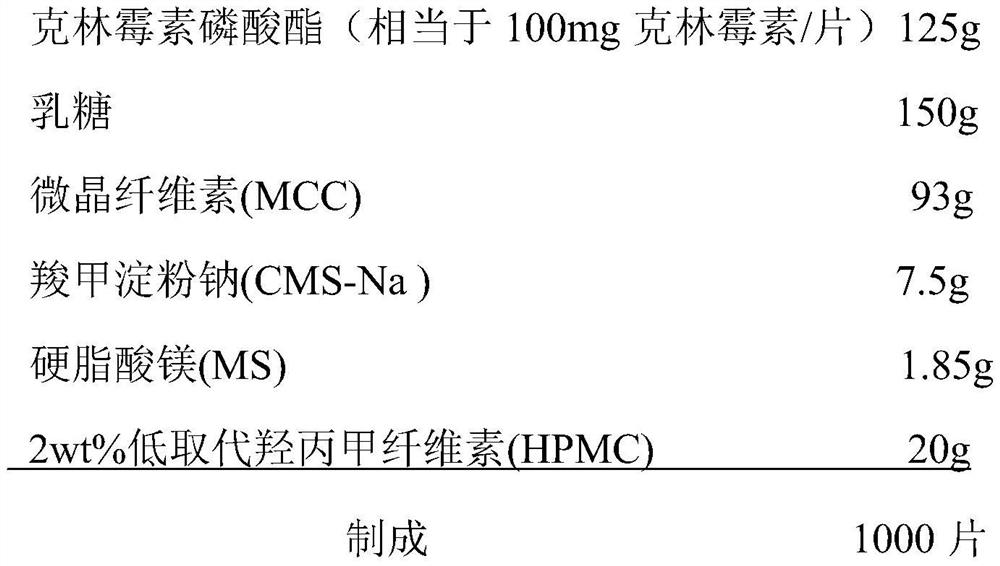
Clindamycin phosphate vaginal tablets and preparation process thereof
ActiveCN112569196AGood swelling rateImprove adhesionAntibacterial agentsOrganic active ingredientsMagnesium stearateStearic acid
The invention provides a clindamycin phosphate vaginal tablet and a preparation process thereof. The clindamycin phosphate vaginal tablet is prepared from the following raw materials in parts by weight: 125 parts of clindamycin phosphate, 140-160 parts of lactose, 90-95 parts of microcrystalline cellulose, 7-8 parts of carboxymethyl starch sodium, 1.5-2.0 parts of magnesium stearate and 10-20 parts of a 1.8-2.2 wt% hydroxypropyl methylcellulose aqueous solution. The clindamycin phosphate vaginal tablet prepared by the preparation method disclosed by the invention is good in swelling rate and relatively large in adhesive force, better exerts the drug effect of clindamycin, shortens the drug use time and improves the use compliance of a patient.
Owner:HAINAN HAISHEN TONGZHOU PHARM CO LTD
Quality control method for cleaning liquor and its derivative formulation for female
ActiveCN1839985AQuality improvementEasy QAHydroxy compound active ingredientsComponent separationLotionAuthentication
The invention discloses a quality control method for women's washing lotion and its derivating agents including bougie, vaginal gelling agent, vaginal tablet and effervescent tablet, characterized in that a process of thin-layer authentication for pomegranate bark and borneo camphor is added, and a high performance liquid phase chromatographic method is employed to control the quality of the medicament.
Owner:江西康美医药保健品有限公司
Rapid dissolve tablet compositions for vaginal administration
InactiveUS20180147152A1Enhance bioadherence of the active ingredientEnhance/sustain systemic absorptionOrganic active ingredientsPill deliverySystemic absorptionPharmaceutical medicine
Disclosed herein are pharmaceutically acceptable rapid dissolve vaginal tablet compositions comprising one or more active pharmaceutical ingredients suitable for therapy via topical action or systemic absorption, and methods of making and using such compositions.
Owner:ADARE PHARM INC
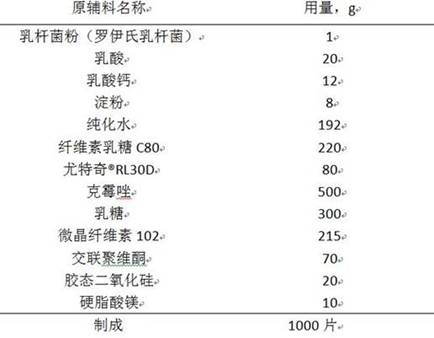
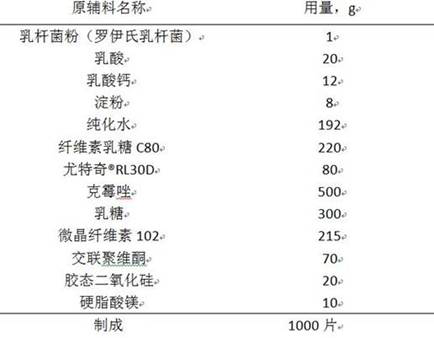
A clotrimazole vaginal tablet composition and preparation method thereof
ActiveCN111789838BImprove complianceGood antibacterial effectAntimycoticsUnknown materialsCelluloseCALCIUM LACTOBIONATE
The invention relates to a live clotrimazole lactobacillus composition and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The clotrimazole Lactobacillus vaginal tablet of the present invention contains 500 mg of clotrimazole; 1 mg of lactobacillus powder; 20 mg to 30 mg of lactic acid; 8 mg to 12 mg of calcium lactate; 6 mg to 12 mg of starch; Tequi®RL30D 66mg~180mg; lactose 225mg~314mg; microcrystalline vitamin 170mg~225mg; crospovidone 70mg; colloidal silicon dioxide 20mg; magnesium stearate 10mg. The invention provides a clotrimazole lactobacillus vaginal tablet.
Owner:DISHA PHARMA GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com