Method for controlling impurities of nifuratel vaginal tablet
A technology of nifuratel and control methods, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve the problems of low specificity and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of impurity control method of nifuratel vaginal tablet, its chromatographic condition is as follows:
[0029] Chromatographic column: Thermo Scientific Hypersil GOLD 250×4.6mm, 5μm
[0030] Column temperature: 30°C
[0031] Injection volume: 20 μl
[0032] Detection wavelength: 210 nm
[0033] Flow rate: 1.0ml / min
[0034] Mobile phase: 0.02% ammonium carbonate solution: acetonitrile = 64:36, volume ratio
[0035] Sample concentration: 0.8 mg / ml
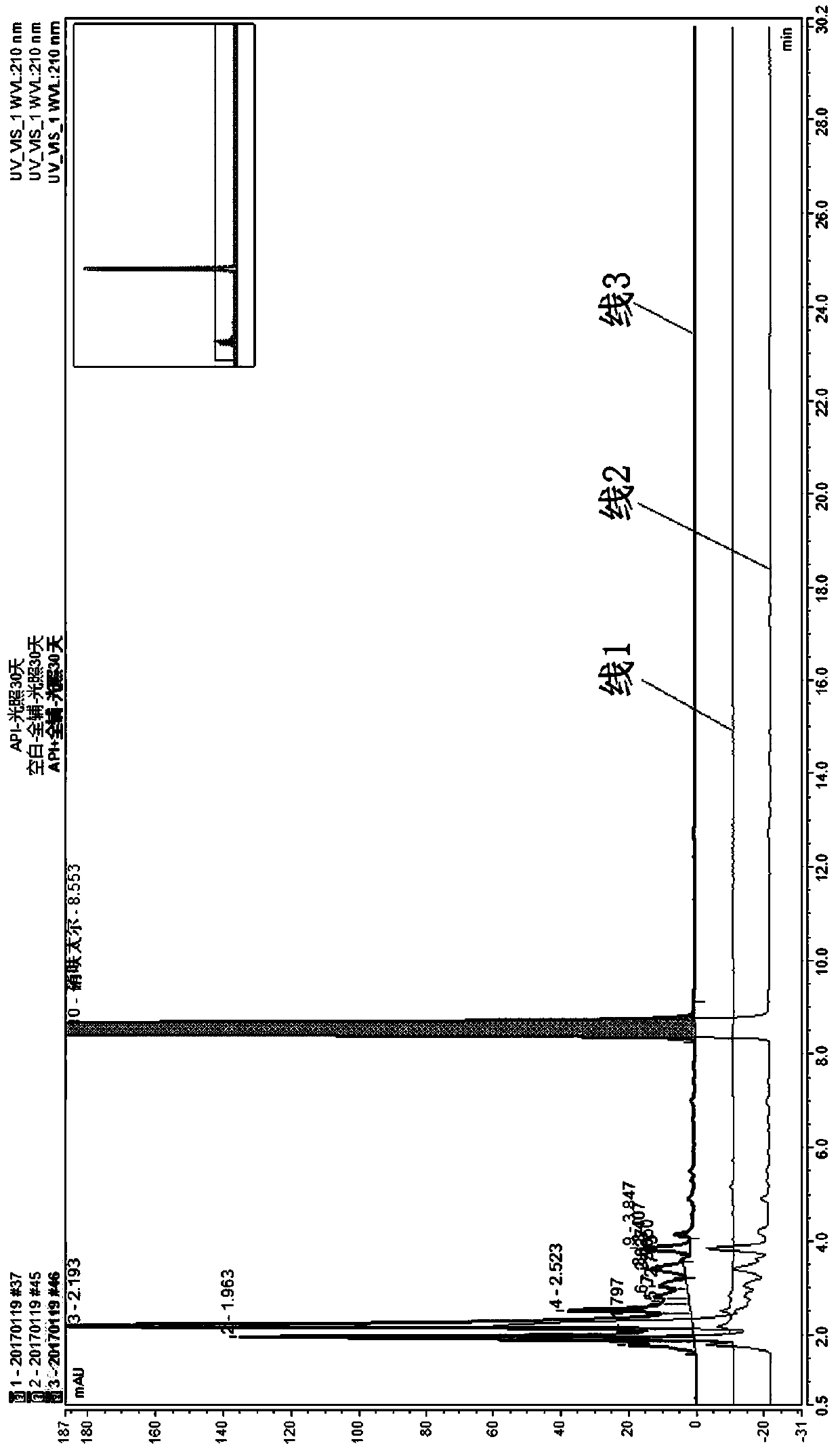
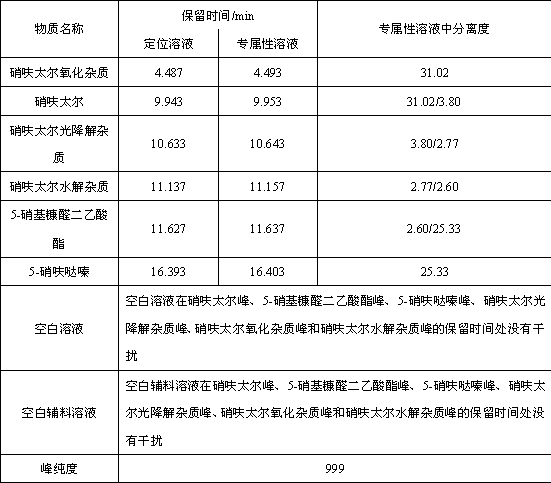
[0036] According to the above chromatographic conditions, the blank solution, nifuratel reference solution, 5-nitrofurfural diacetate reference solution, 5-nifuridazine reference solution, auxiliary material blank-30 days light solution, nifurate Inject one injection of Taier-30-day light solution and nifuratel+full excipients-30-day light solution.
[0037] The peak shapes of nifuratel, 5-nitrofurfural diacetate and 5-nifuridazine were all good, and the mutual separation was good. Therefore, the column is suitab...
Embodiment 2
[0039] A kind of impurity control method of nifuratel vaginal tablet, its chromatographic condition is as follows:
[0040] Chromatographic column: Thermo Scientific Hypersil GOLD 250×4.6mm, 5μm
[0041] Column temperature: 30°C
[0042] Injection volume: 20 μl
[0043] Detection wavelength: 260 nm
[0044] Flow rate: 1.0ml / min
[0045] Mobile phase: 0.02% ammonium carbonate solution: acetonitrile = 64:36, volume ratio
[0046] Sample concentration: 0.8 mg / ml
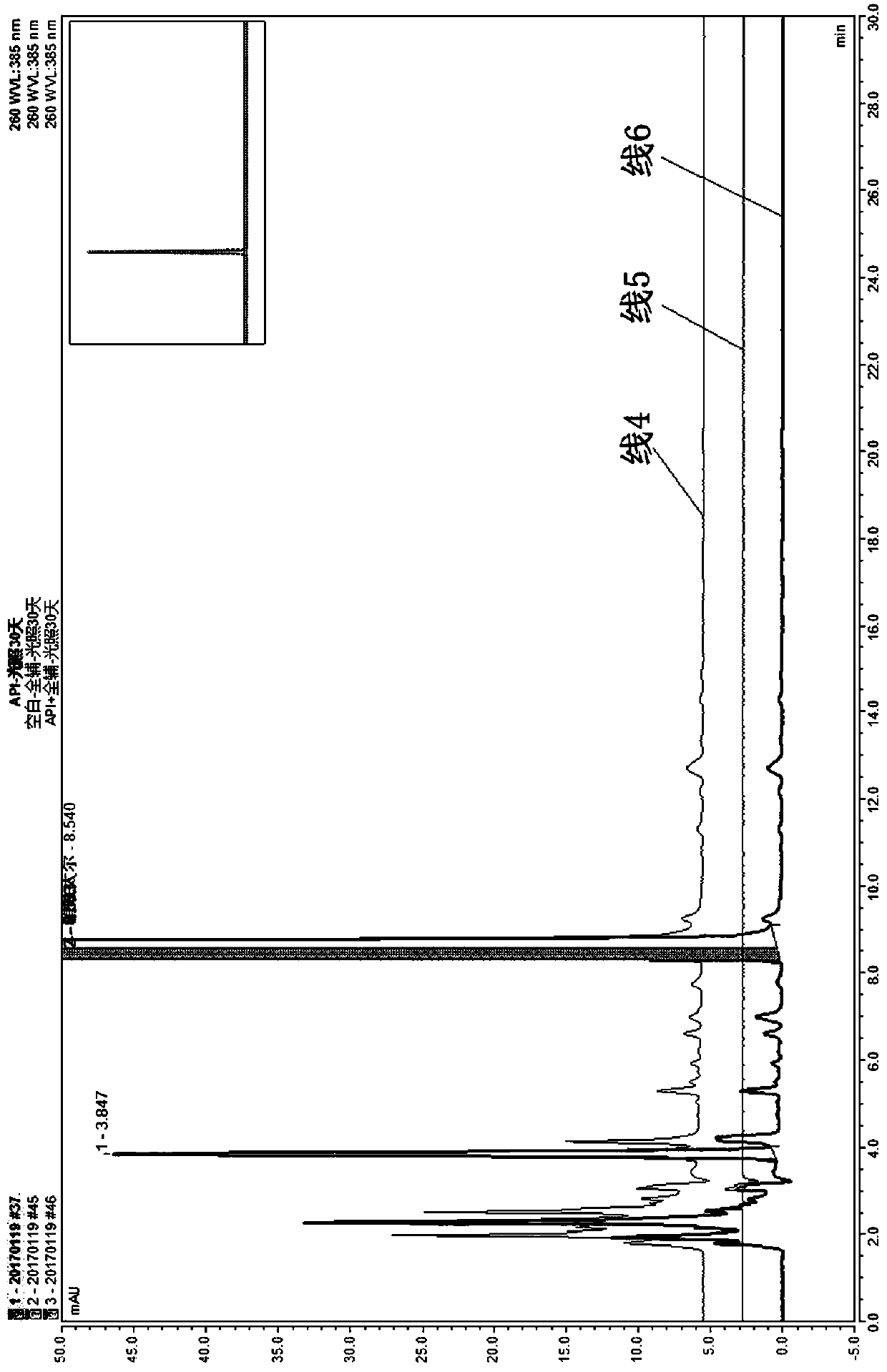
[0047] According to the results of Example 1, the blank of the excipient produces an interference peak at the retention time of the photodegradation impurity of nifuratel. Therefore, try to adjust the detection wavelength to avoid the appearance of interference peaks. When the detection wavelength was changed to 260nm, the blank of excipients did not produce interference peaks at the photodegradation impurities of nifuratel. At 210nm and 260nm, the impurity profile is consistent and the number of impurities is co...
Embodiment 3
[0049] A kind of impurity control method of nifuratel vaginal tablet, its chromatographic condition is as follows:
[0050] Chromatographic column: Thermo Scientific Hypersil GOLD 250×4.6mm, 5μm
[0051] Column temperature: 30°C
[0052] Injection volume: 20 μl
[0053] Detection wavelength: 260 nm
[0054] Flow rate: 1.0ml / min
[0055] Mobile phase system: Phase A: Phase B = 64:36, volume ratio
[0056] Phase A: 10 mM KH 2 PO 4 , adjust the pH to 3.00 with phosphoric acid
[0057] Phase B: Acetonitrile
[0058] Sample concentration: 1.0 mg / ml
[0059] When the sample concentration was adjusted to 1.0mg / ml, the quantitative limit of the method could reach 0.03%, and the retention times of nifuratel, 5-nitrofurfural diacetate and 5-nifuridazine were 9.4min, 13.2min and 20.9min, RRT were 1.00, 0.20 and 0.78, indicating that the specificity of the method was good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com