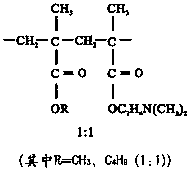Preparation method of clotrimazole vaginal tablets
A technology for vaginal tablets and clotrimazole, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problem of reducing product impurity levels, unstable clotrimazole, and unconcerned impurities and product acidity and other issues, to achieve the effect of industrialized production, migration prevention and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
1000 pieces
[0031] Preparation Process:
[0032] ① Preparation of binder solution for wet granulation: Dissolve the prescribed amount of lactic acid in an aqueous solution of hypromellose.
[0033] ②Preparation of granule coating liquid: add sodium lauryl sulfate, stearic acid and Eudragit E PO in the ratio of 10:1:1.5, and then add appropriate amount of water to make Eudragit E PO with a concentration of 10%. The coating liquid is homogeneous and uniform.
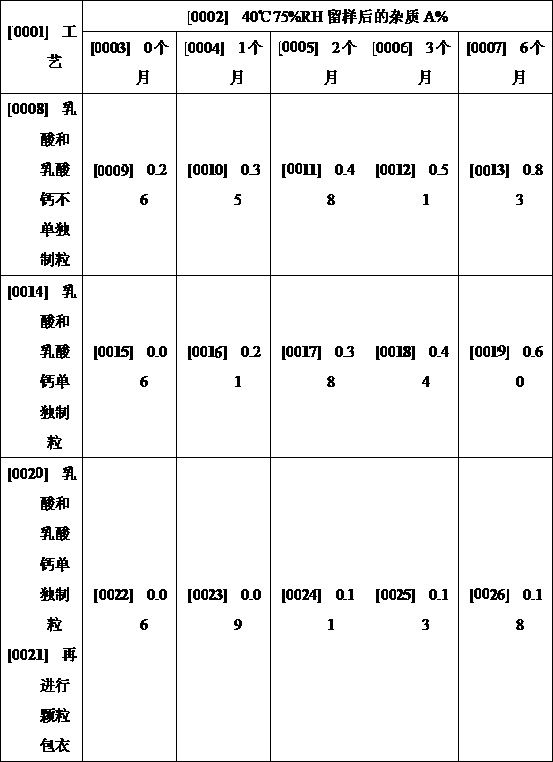
[0034] ③Preparation of lactic acid and calcium lactate granules: Place the prescribed amount of calcium lactate and cornstarch in a fluidized bed and mix evenly, granulate with the binder solution in step ①, dry, and coat the granules with the coating solution in step ②. For coating, the material temperature is 25-30°C, the amount of coating liquid is 40% of the weight of the substrate, after the liquid spraying, reduce the degree of fluidization, dry at 30°C for 5 minutes, and then dry in an oven at 40°C for 2 ho...
Embodiment 5
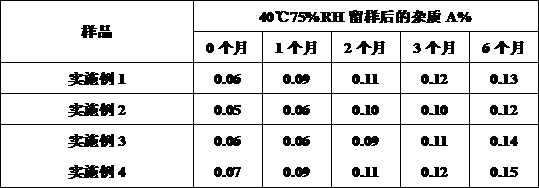
[0059] The samples of Examples 1-4 were kept as stability samples, and the change of impurity A was determined.
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com