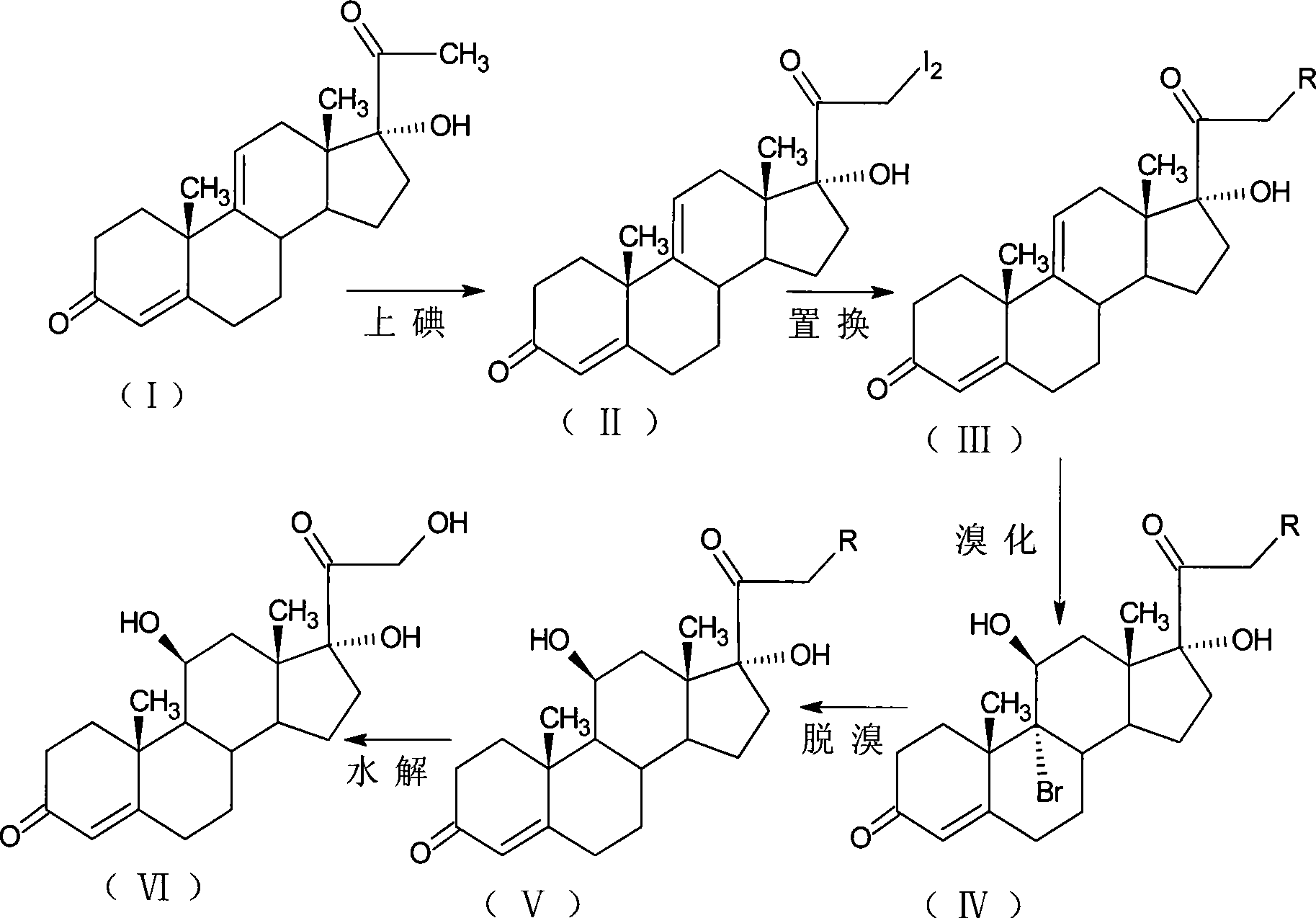
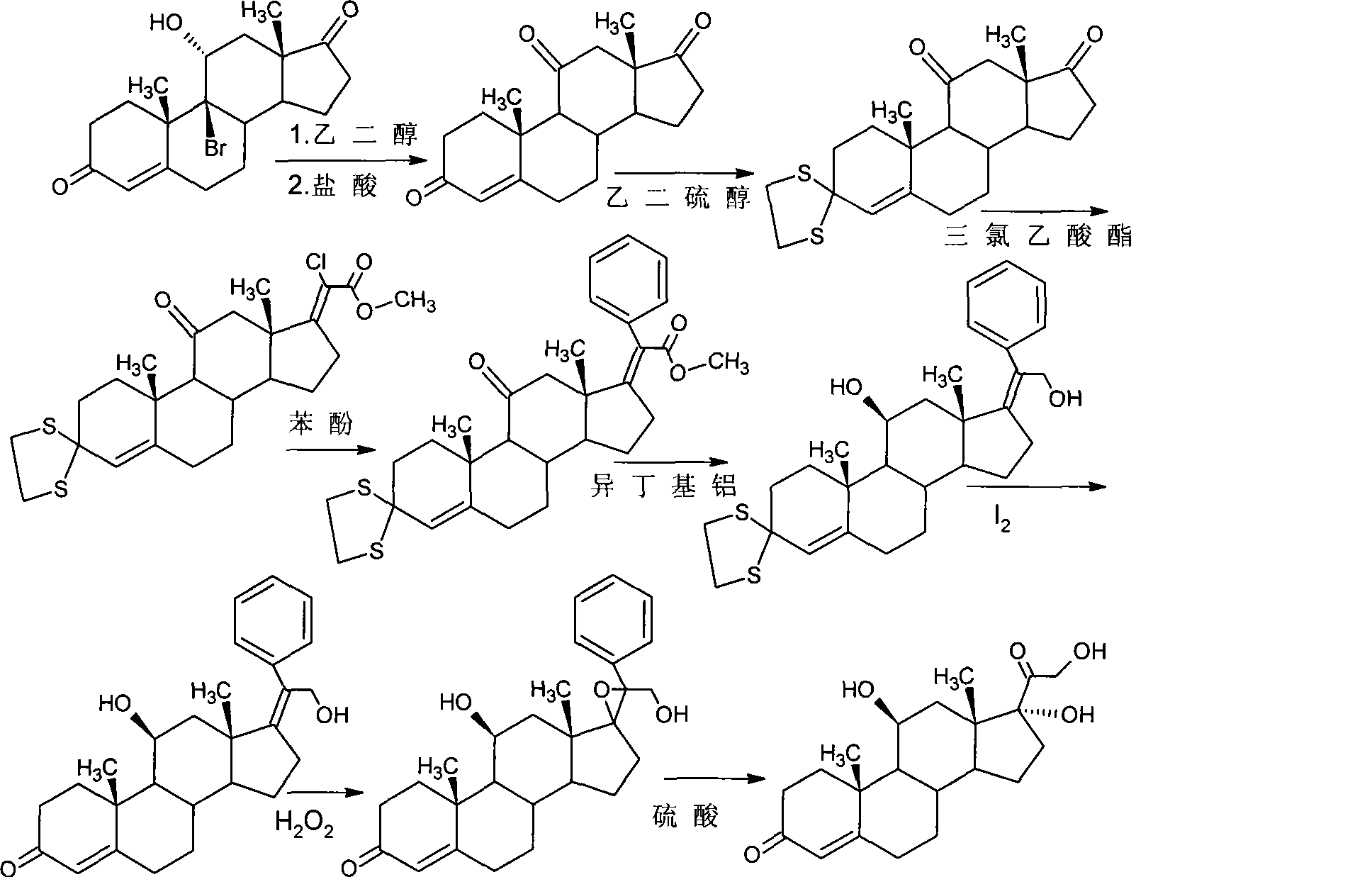
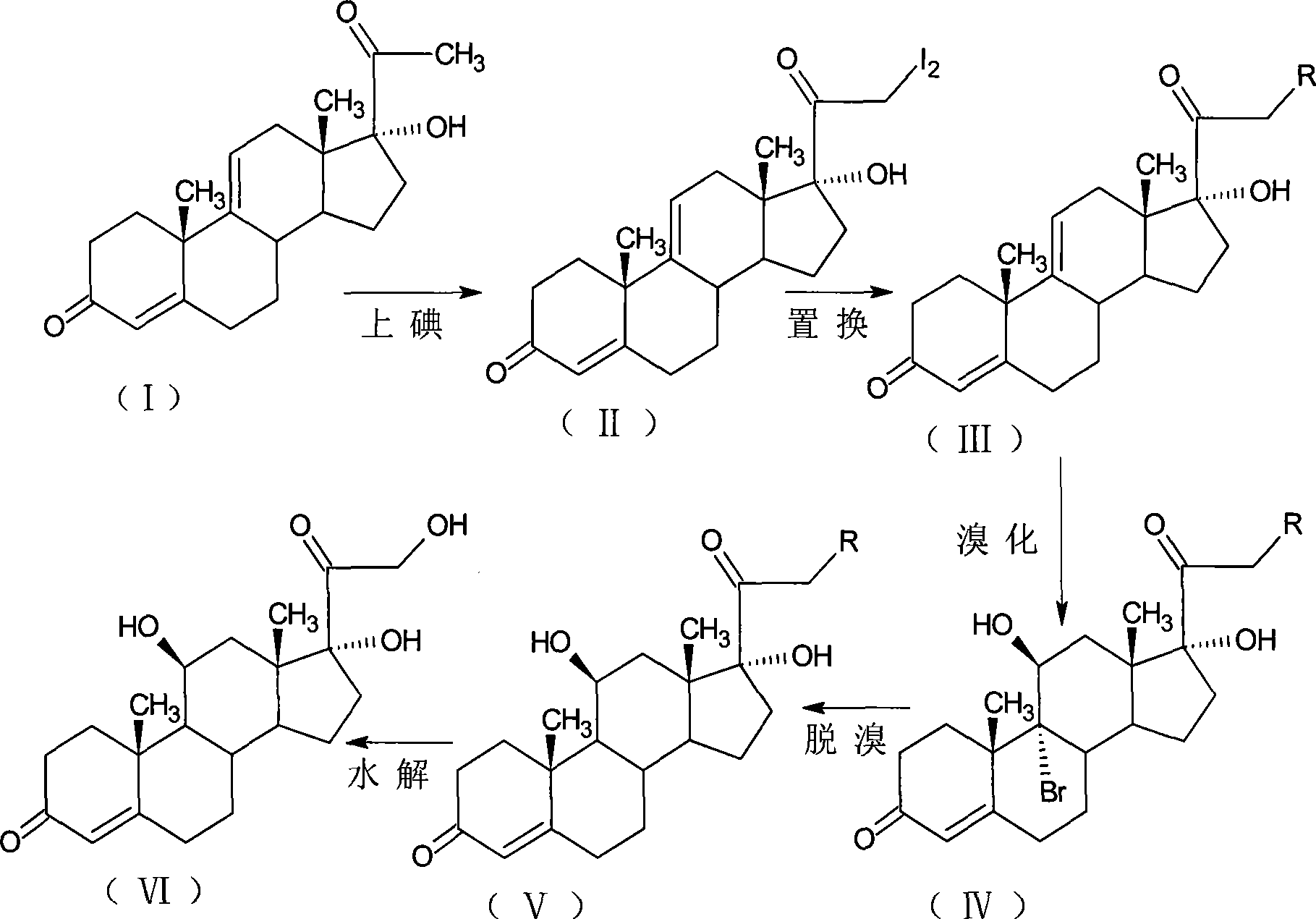
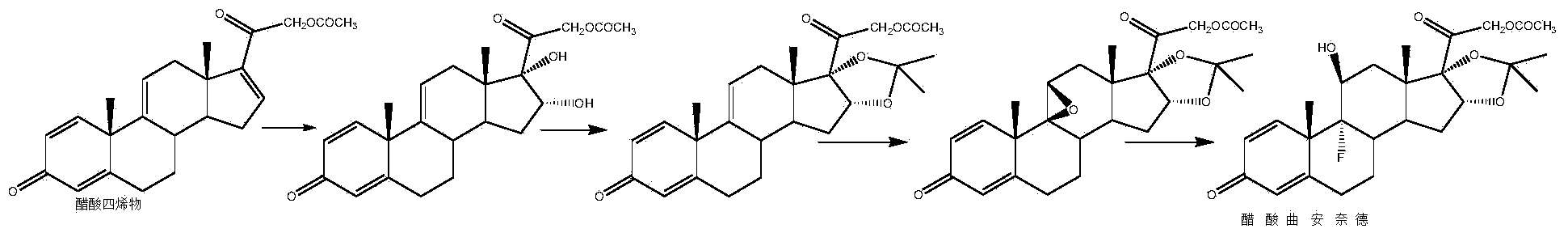
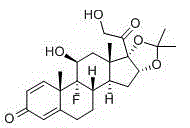
Patents
Literature
170 results about "Triamcinolone acetonide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a variety of skin conditions (e.g., eczema, dermatitis, allergies, rash).
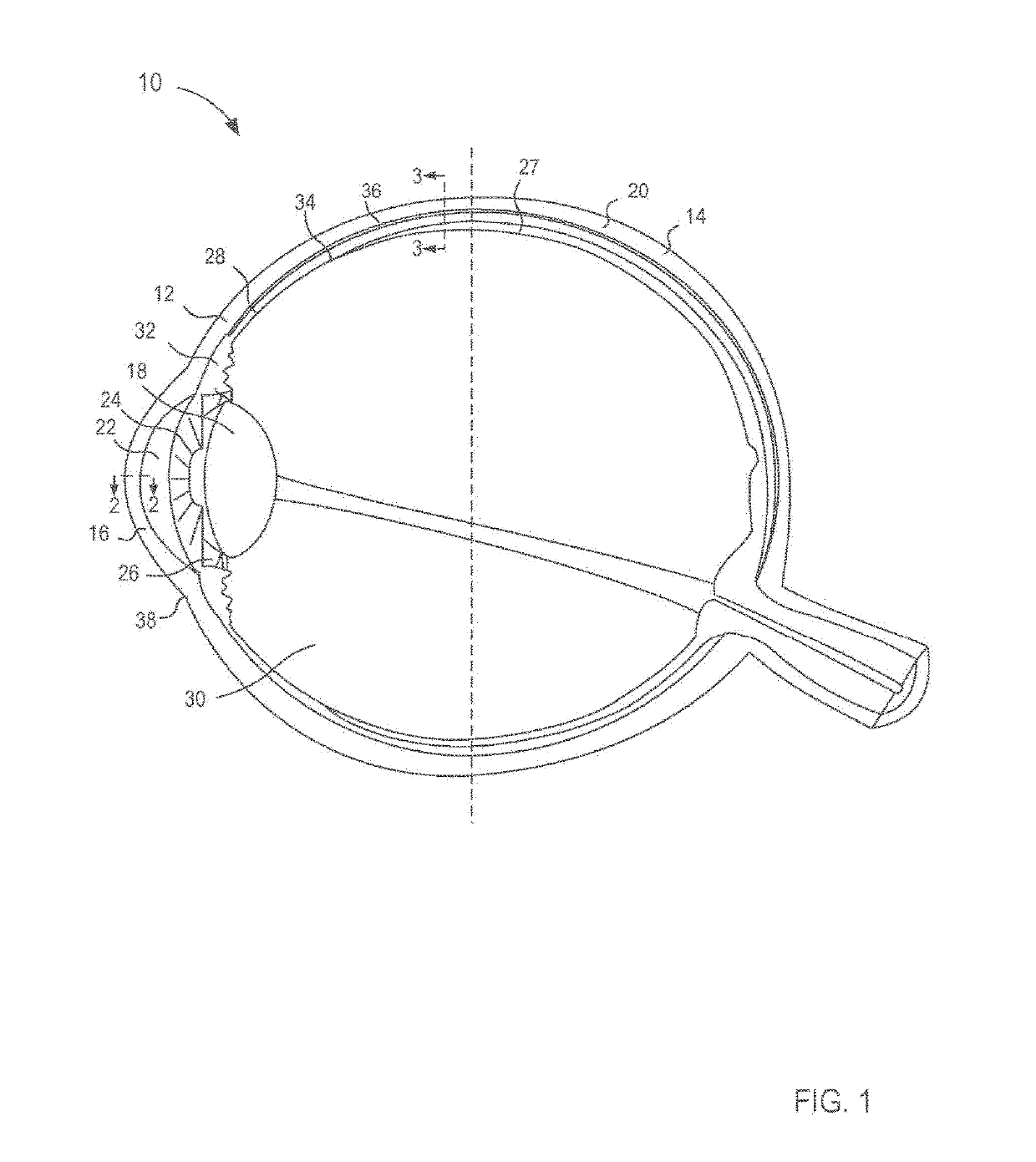
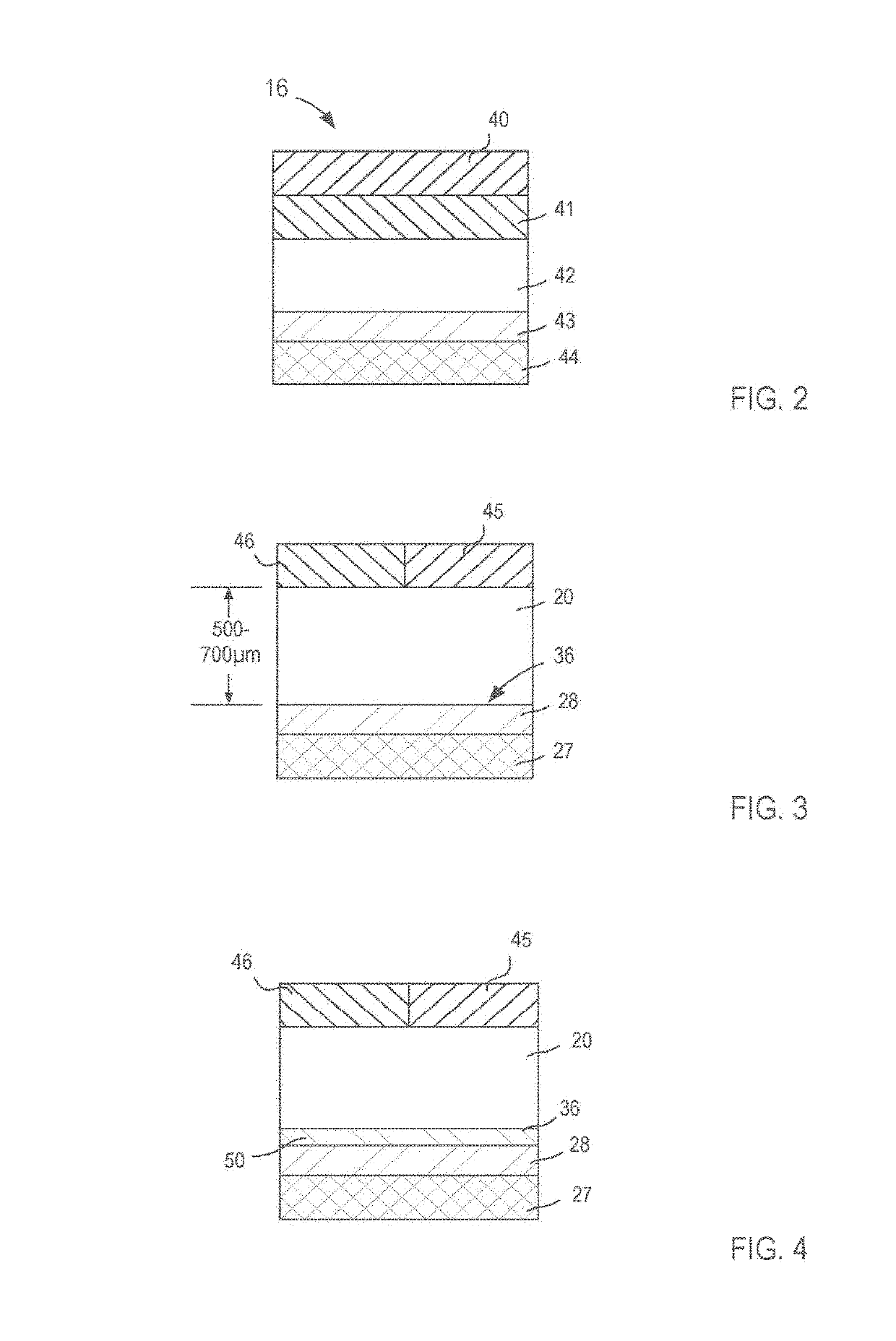
Ophthalmic compositions and methods for treating ophthalmic conditions
InactiveUS20060009498A1Delayed extended releaseEnhance and improve visionBiocideSenses disorderFluid compositionSolvent
Compositions, and methods of using such compositions, useful for placement, for example injection, into the interior of human or animal eyes are provided. Such compositions include a therapeutic component, such as one or more corticosteroids, a biocompatible polymeric component, and a solvent component. The composition is in a fluid form before placement in the interior of an eye, and becomes less fluid after the composition is placed in the eye to form an extended or delayed release drug delivery element or system. The drug delivery element is formed by the dissipation of the solvent from the composition when the composition is placed in the interior of an eye. One example of a composition includes triamcinolone acetonide as a therapeutic agent. A method of treating an ophthalmic condition, or otherwise improving or enhancing vision of a patient, comprises placing the fluid composition in the interior of the eye. The method may be practiced by injecting the fluid composition into the interior of the eye.
Owner:ALLERGAN INC
Synergetic composition for the treatment of psoriasis and other skin disorders and method therefor
InactiveUS20030185915A1ThinningSuppression problemBiocideUnknown materialsBULK ACTIVE INGREDIENTParapsoriasis
Synergetic compounded medication formula for the treatment of psoriasis, seborrhea, dermatitis, dandruff, eczema, acne, and other skin disorders. The present invention is to provide regenerative treatment of skin disorders recurrent in all areas of the body. The invention of this disclosure uses a well-known corticosteroid as an active ingredient, namely Triamcinolone acetonide, which when used in combination with a special formula is effective, easy to use, and less expensive than similar products available with a prescription in the market. A method for administering said composition to inhibit proliferation of psoriatic cell populations in the epidermis is disclosed
Owner:CARLO JAIME +3
Preparation of hydrocortisone and derivatives thereof
The invention relates to a preparation method of steroids compounds, in particular to the preparation of hydrocortisone and derivatives thereof. The invention adopts 17-hydroxy-4, 9-diene-pregna-3, 20-diketone as the original material which is modified by 21 bits and 9, 11 bits so as to obtain anecortave acetate retaane, the hydrocortisone and hydrocortisone acetate, and the like. The technical process of the invention adopts the existing intermediates of manufacturers as the original material; the route is simple; the feasibility is high; the operability is strong; the materials are available; the use of expensive accessories is avoided so as to largely reduce the cost of industrial manufacturing; a plurality of products can be obtained on the same manufacturing line; the yield and the cost are dramatically better than that of the prior methods used for synthesizing the hydrocortisone and the derivatives thereof; moreover, the adoption of the existing intermediates realizes the combined-line production of triamcinolone acetonide series products, hydrocortisone series products and anecortave acetate series products so as to greatly reduce the manufacturing cost and the conditions for industrial manufacturing; wherein, R is equal to minus OCOR1 and R1 is equal to the alkyl with the carbon less than 11.
Owner:TIANJIN PHARMA GROUP CORP
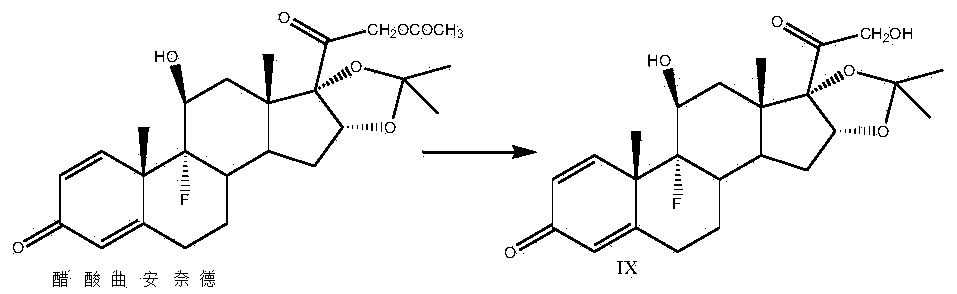
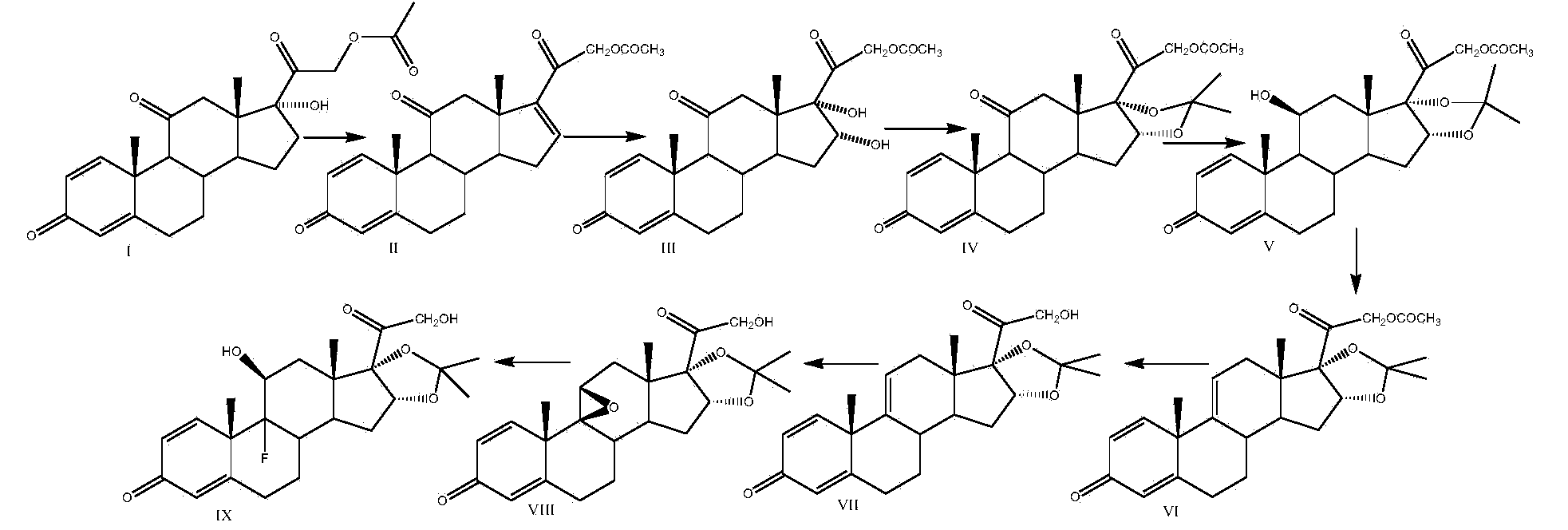
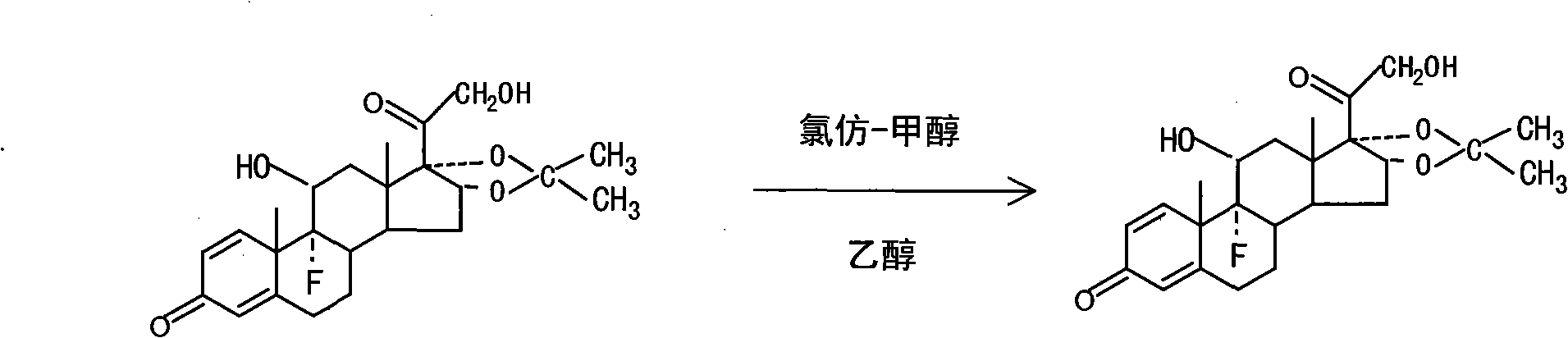
Preparation method of triamcinolone acetonide
The invention discloses a preparation method of triamcinolone acetonide. A compound I prednisone acetate which is taken as a starting material is subjected to a primary elimination reaction, an oxidation reaction, a condensation reaction, a reduction reaction, a secondary elimination reaction, a hydrolysis reaction, an epoxidation reaction and a fluorination reaction, so that an end-product IX, 9-fluoro-11beta, 21-dihydroxyl-16a,17-[(1-methyl ethylidene) bi(oxo)]-pregnen steroid-1,4-diene-3, 20-diketone, namely triamcinolone acetonide, is obtained. The preparation method of the triamcinolone acetonide has the advantages that a relatively cheap starting raw material is adopted, each reaction step can be relatively easily realized, and yield is relatively high, so that production is more economic and safer, and the triamcinolone acetonide is more applicable to industrial production; a multi-step protection and deprotection operation is simplified, a synthetic route is greatly shortened, and production cost is reduced; possibility that a three-position carbonyl group is reduced in a reduction process is almost zero, so that side effects are greatly reduced, and yield and quality of the reduction reaction are improved.
Owner:江西赣亮医药原料有限公司
Medicament compound for treating skin disease
InactiveCN101199787ANo absorptionNo side effectsHeavy metal active ingredientsAnthropod material medical ingredientsDiseaseDEXAMETHASONE TABLETS
The invention discloses a drug combination for treating dermatosis. The combination is prepared by the following raw materials with certain parts by weight: Galla Chinensis 6-12, Cortex Pseudolaricis 12-24, Radix Sophorae Flavescentis 15-30, Cortex Phellodendri 12-24, Fructus Kochiae 15-30, powdered Sulphur 10-20, Radix Isatidis 8-16, Radix Stemonae 10-20, powdered Calamina 12-24, Hibiscus mutabilis L 8-16, Typhonium gigante um Engl. 10-20, pure Realgar 15-26, Rhizoma Polygonati 10-15, Cinnabaris 8-14, Borneolum, Dexamethasone Tablets 0.25-0.5 or Triamcinolone Acetonide Tablets 0.25-0.5, and Vaseline 1000-2000. The drug combination of the invention belongs to the external-use skin liniment. Years of tests prove that the invention has the advantages of effective and deep disinfection, quick effect and more than 90 percent of effective rate. The invention is applicable for treatment of colpomycosis, mycotic vaginitis and Candida albicans infection; for disinfection and sterilization of skin sensibility and pruritus; and for treatment of foot and hand tinea, tinea manuum, dermatophytosis and balanitis.
Owner:朱锋
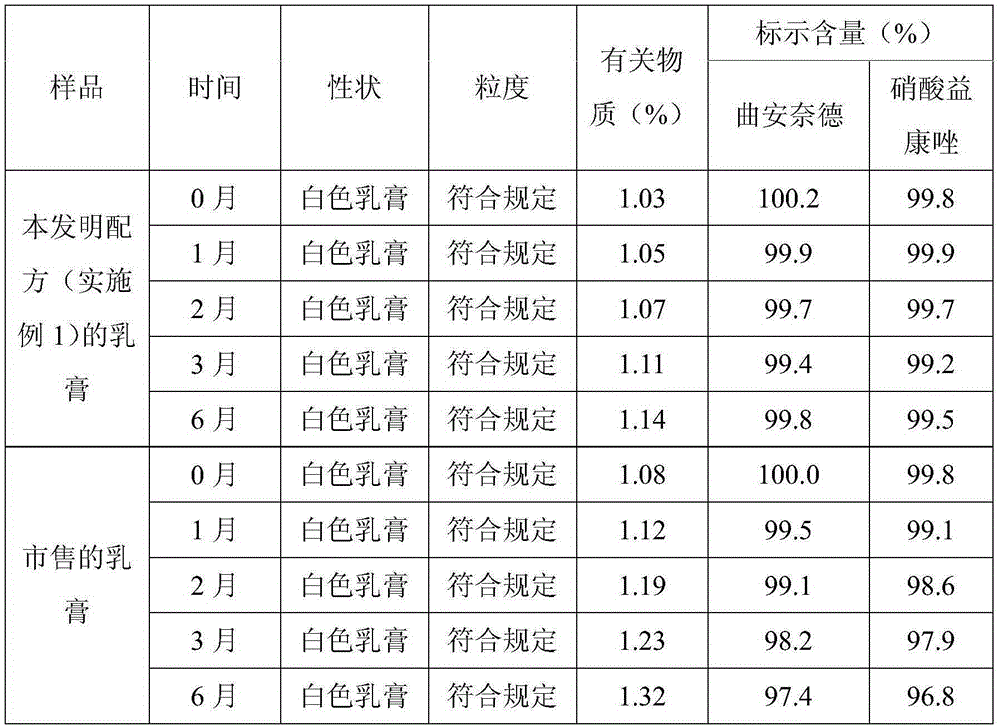
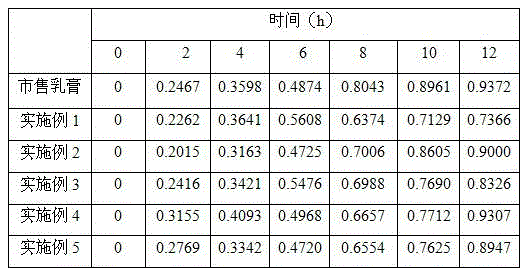
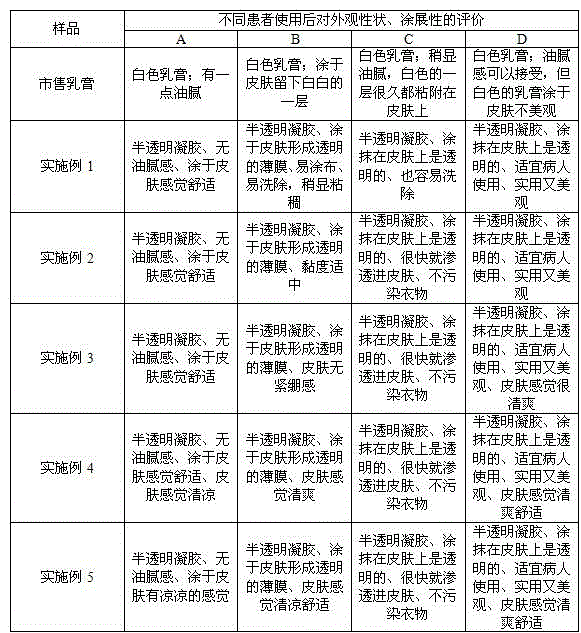
Triamcinolone acetonide econazole cream
InactiveCN101239066ASmall granularityNo smellOrganic active ingredientsAntimycoticsAlcoholIrritation
The invention discloses a triamcinolone acetonide and econazole nitrate cream which contains econazole nitrate and triamcinolone acetonide. The invention is characterized in that the cream also contains octodecyl alcohol or hexadecanol. The triamcinolone acetonide and econazole nitrate cream is more subtle and even, is easy to be daubed and washed on and away from the skin and is harmless to skin, has no odor. No greasiness is to be felt when the cream is daubed on the skin, and the quality is more stable.
Owner:刘全胜
Low viscosity, highly flocculated triamcinolone acetonide suspensions for intravitreal injection
ActiveUS8128960B2Improved stability characteristicsEasily resuspendedPowder deliveryOrganic active ingredientsViscosityTriamcinolone acetonide
Triamcinolone acetonide suspension compositions are disclosed. The suspension compositions have a relatively low viscosity and are easy to extrude through a 27- or 30-gauge needle but are highly flocculated and easily redispersed. The compositions are particularly suitable for intravitreal injection.
Owner:NOVARTIS AG
Diagnostic method and kit for implantation of a sustained release drug-delivery implant with a steroid
InactiveUS20050226814A1Minimally invasiveReduce swellingCompounds screening/testingOrganic active ingredientsSustained release drugMedicine
The present invention is a method of the present invention screens a patient with macular edema associated with visual loss for implantation of a sustained release drug-delivery implant that is less invasive than implanting a drug-delivery implant and is minimally invasive. The method comprises injecting a first steroid, eg. triamcinolone acetonide, having anti-inflammatory activity as a bolus into a patient's eye. Patients that experience a reduction in swelling in the retina (typically the macula and preferably the fovia) and improved vision are identified as candidate patients. Candidate patients are patients that are more likely to benefit from the implantation of a sustained release drug-delivery implant into the eye containing a second steroid, for example fluocinolone acetonide.
Owner:BAUSCH & LOMB INC
Aqueous-based pharmaceutical composition
InactiveUS7122206B2Satisfied with stabilitySatisfactory shelf-life propertyOrganic active ingredientsPowder deliveryNasal passageNasal cavity
An aqueous pharmaceutical composition which is capable of being sprayed into the nasal cavity of an individual and which comprises: (A) a pharmaceutically effective amount of solid particles of medicament which is effective in treating a bodily condition by virtue of its being present on the mucosal surfaces of the nasal cavity; and (B) a suspending agent in an amount effective to maintain said particles dispersed uniformly in the composition and to impart to the composition the following thixotropic properties: (i) the viscosity of the position in unsheared form is relatively high, with the composition being in gel-like form; (ii) as the composition is subjected to shear (shaken) in preparation for spraying, the viscosity of the composition becomes relatively low and such that the composition in the form of a mist flows readily into the nasal passages for deposit on the mucosal surfaces of the nasal cavity; and (D) in deposited form on the mucosal surfaces, the viscosity of the composition is relatively high and such that it resists being cleared from the mucosal surfaces by the inherent mucocillary forces which are present in the nasal cavity, a method of use of the composition and a method for preparation of the composition, including in preferred form the use of anti-inflammatory steroid, for example, triamcinoline acetonide, and an odorless form of the composition.
Owner:AVENTISUB LLC
Anti-scar drug film and its preparing method
InactiveCN1729986APromote precipitationEvenly dispersedSynthetic polymeric active ingredientsDermatological disorderSilicone oilScars
The invention provides a cicatrix-resisting membrane comprising a medicinal membrane, wherein the medicinal membrane is a silicon gel film containing medicament of triamcinolone acetonide acetate and silica oil. The invention also discloses the process for preparing the membrane.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Aerosol pharmaceutical solution formulation containing glucocorticoids stable to the storage; method for stabilizing formulations and use of a stabilizer
Aerosol pharmaceutical solution formulations containing glucocorticosteroids stabilized by adding water or a mixture of water and citric acid, avoiding corrosion of the elements of container under standard storage conditions are described. The formulations comprise: between 0.05 and 1.0% by weight of a glucocorticoid having a C-20 ketone and OH group in carbons 17 and / or 21 as active substance; between 0.10 and 3% by weight of a selected stabilizer selected between water, or a mixture of water and organic acid selected between citric acid and tartaric acid; a cosolvent in amount sufficient to solubilize the active substance; optionally a surfactant; and propellant in sufficient amount to achieve100% by weight of the finished solution. Glucocorticosteroids having a C-20 ketone and an OH group at the C-17 and / or 21 position with varying substituents, have many well-known therapeutic uses, especially based upon their anti-inflammatory activity. This types of steroids, glucocorticosteroids, and their pharmaceutical formulations are useful in the treatment of several diseases including bronchial disorders and inflammatory conditions. Preferably, the glucocorticoid is selected between Triamcinolone Acetonide, budesonide, Dexamethasone and betamethasone 17-valerate. A method for stabilizing aerosol pharmaceutical solution formulations containing glucocorticoids susceptible to oxidative degradation and use of a stabilizer selected between water and a mixture of water and organic acid selected between citric acid and tartaric acid are also described
Owner:LAB PABLO CASSARA
Triamcinolone acetonide and econazole cream and preparation method thereof
ActiveCN103860566AImprove stabilityFast transdermal absorptionOrganic active ingredientsAntimycoticsCellulosePolythylene glycol
The invention discloses triamcinolone acetonide and econazole cream and a preparation method thereof. The cream is prepared by uniformly mixing a water phase and an oil phase, wherein the water phase is a hydroxy propyl cellulose water solution and the oil phase is prepared by virtue of the following steps: dissolving triamcinolone acetonide and econazole in isopropyl myristate, then adding dodecylicpolyethylene glycol glyceride laurate and polyethylene glycol 400 and uniformly stirring. Compared with the prior art, the medicament disclosed by the invention exists in the cream in a nanometer form, and is high in stability, rapid in transdermal absorption, simple in preparation process, free of use of complicated emulsion preparation equipment and suitable for the requirements of large-scale production.
Owner:江苏晨牌邦德药业有限公司
Compositions and methods for treating noninfectious uveitis
InactiveUS20190269702A1Reduce inflammationReduces inflammatory scoreOrganic active ingredientsSenses disorderUveitisMacular edema
The present invention relates to methods, devices, and compositions for treating ocular disorders such as uveitis, macular edema associated with uveitis, and diabetic macular edema. For example, the methods include treatment of subjects having macular edema associated with non-infectious uveitis, or diabetic macular edema, by administering to the subjects a triamcinolone composition via non-surgical administration to the suprachoroidal space (SCS) of the eye.
Owner:CLEARSIDE BIOMEDICAL
Glucocorticosteroid and chemotherapy medicament carried by anticancer sustained-release agent
InactiveCN101502484AInhibition formationImprove permeabilitySolution deliveryPharmaceutical non-active ingredientsGlucocorticoidPolyethylene glycol
The invention provides an anti-cancer sustained-release agent co-carrying glucocorticoid and chemotherapeutic drugs and belongs to sustained-release injections. The anti-cancer sustained-release agent comprises sustained-release microspheres and a solvent, wherein, the sustained-release microspheres comprise anti-cancer active components and sustained-release auxiliary materials; and the solvent is a particular solvent containing a suspending agent. The glucocorticoid is selected from prednisolone, methylprednisolone, dexamethasone, betemethasone, triamcinolone acetonide or triamcinolone acetonide; the chemotherapeutic drugs are selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogues and the like; the sustained-release auxiliary materials are biocompatible high-polymers, such as polylactic acid and the copolymers thereof, polyethylene glycol, carboxyl-terminated polylactic acid copolymers, polyfatty acid dimer-sebacic acid copolymers, poly(erucic acid dimer-sebacic acid), poly(fumaric-co-sebacic acid), polifeprosan and the like; and the suspending agent with the viscosity being 100cp to 3,000cp (at the temperature of 20 to 30 DEG C) is selected from sodium carboxymethyl cellulose and the like. The anti-cancer active components and the sustained-release microspheres can further be prepared into sustained-release implants which can effectively inhibit the growth of tumors, alleviate edema and improve the curative effects of radiotherapy and chemotherapy by intra-tumor or peri-tumor injection or placement.
Owner:SHANDONG LANJIN PHARMA
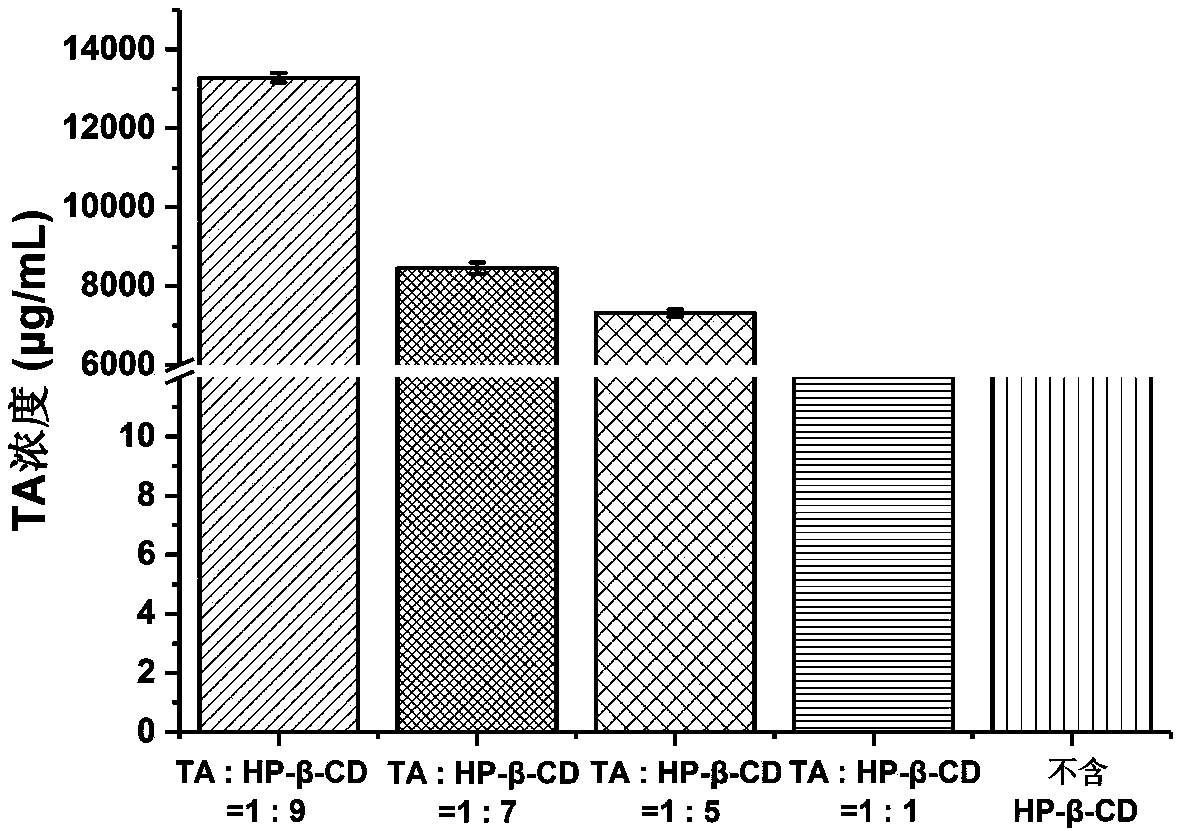
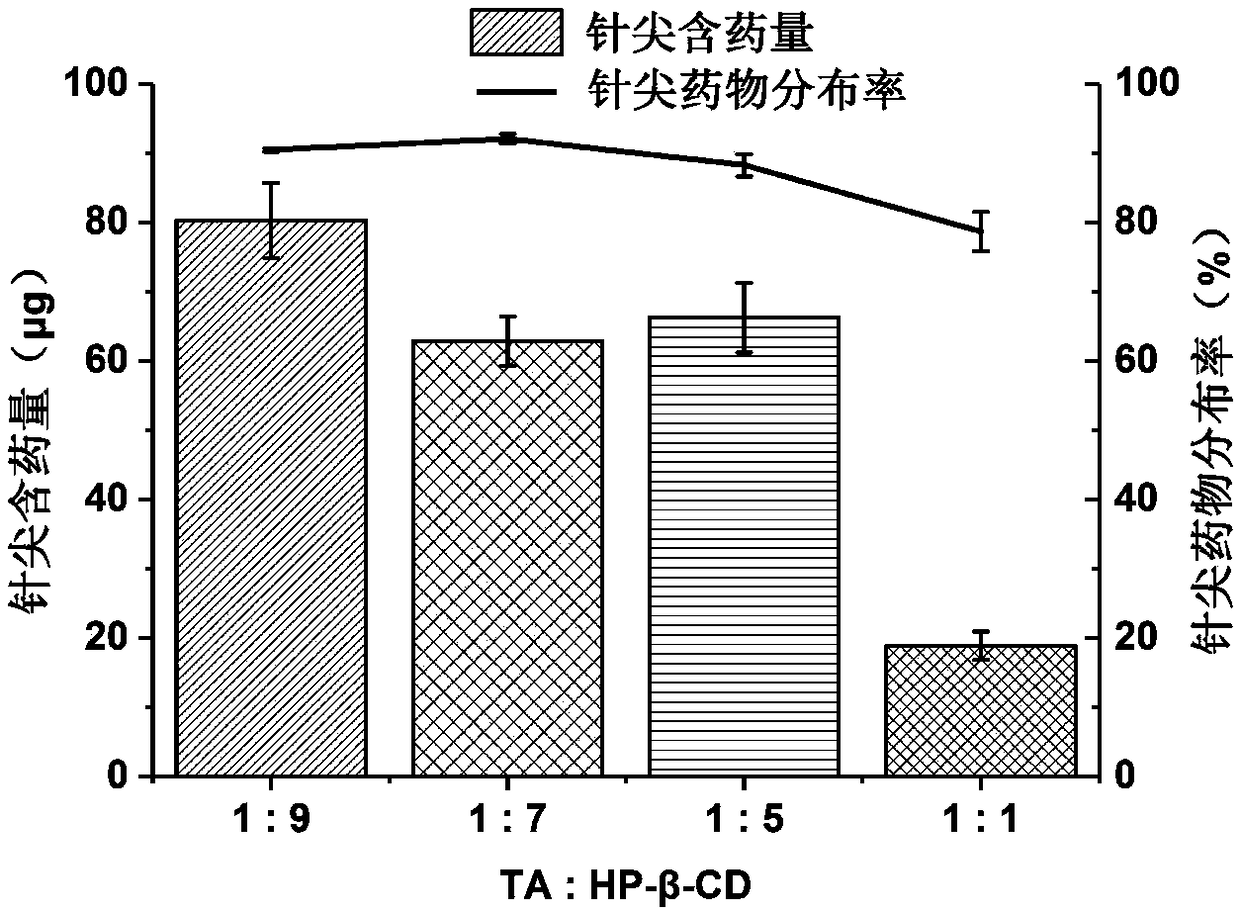
Triamcinolone acetonide soluble micro-needle and preparation method thereof
InactiveCN108403617AReduced risk of penetration into subcutaneous tissueRelieve painOrganic active ingredientsMicroneedlesBeta-CyclodextrinsMicro-needle
The invention relates to a triamcinolone acetonide soluble micro-needle and a preparation method thereof. The soluble micro-needle comprises a needle tip and a substrate, the needle tip comprises sodium hyaluronate, triamcinolone acetonide and beta-cyclodextrin, the molar ratio of triamcinolone acetonide to the beta-cyclodextrin is 1:5-10, the weight ratio of the sodium hyaluronate to the triamcinolone acetonide is 15-70:1, and the substrate comprises high-molecular polymers. According to the triamcinolone acetonide soluble micro-needle, the medicine content of the needle tip is high, the needle tip has excellent mechanical strength and can penetrate scar tissues harder and more compact than common skins, a medicine delivery channel is formed, medicines are released, the micro-needle can effectively replace a current mode that the medicines are delivered by a medical syringe, pain caused by injection of the syringe is greatly reduced, and the medicines are efficiently delivered. The soluble micro-needle is prepared by a three-step centrifugation forming method, and the medicine loading ratio of the needle tip can be greatly increased.
Owner:SUN YAT SEN UNIV
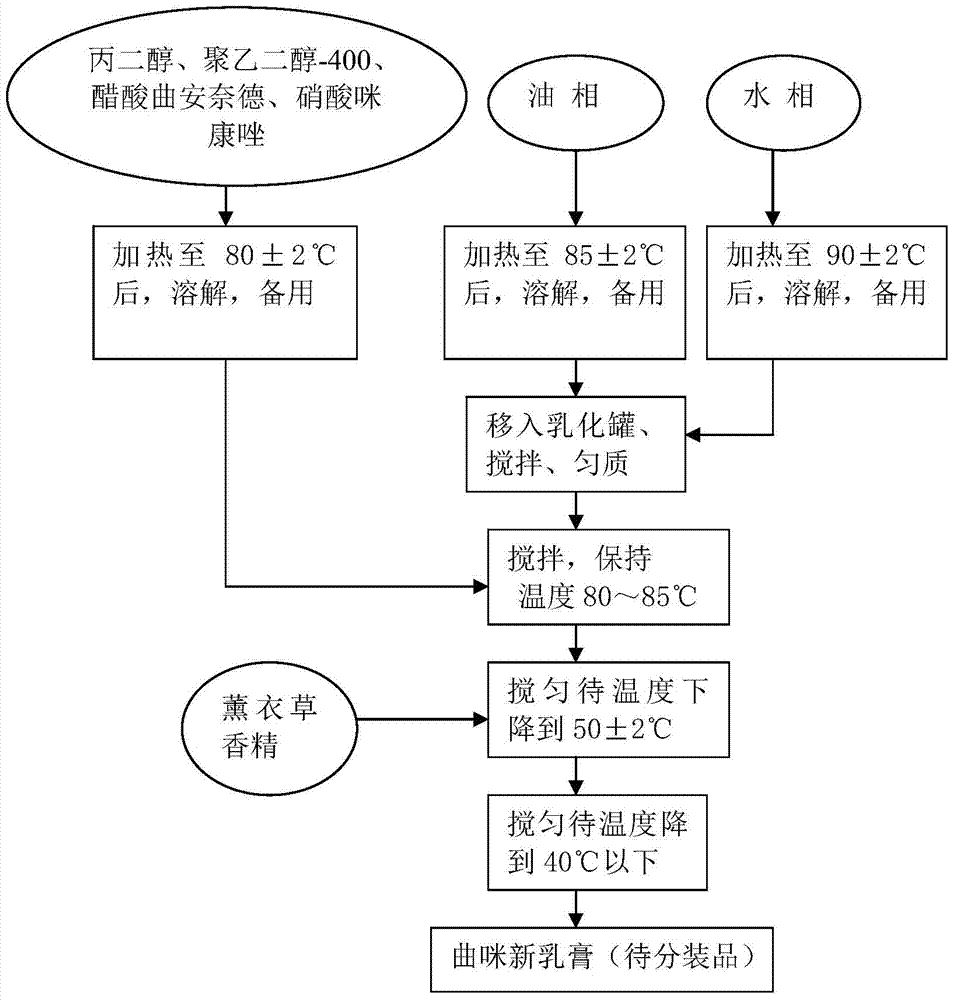
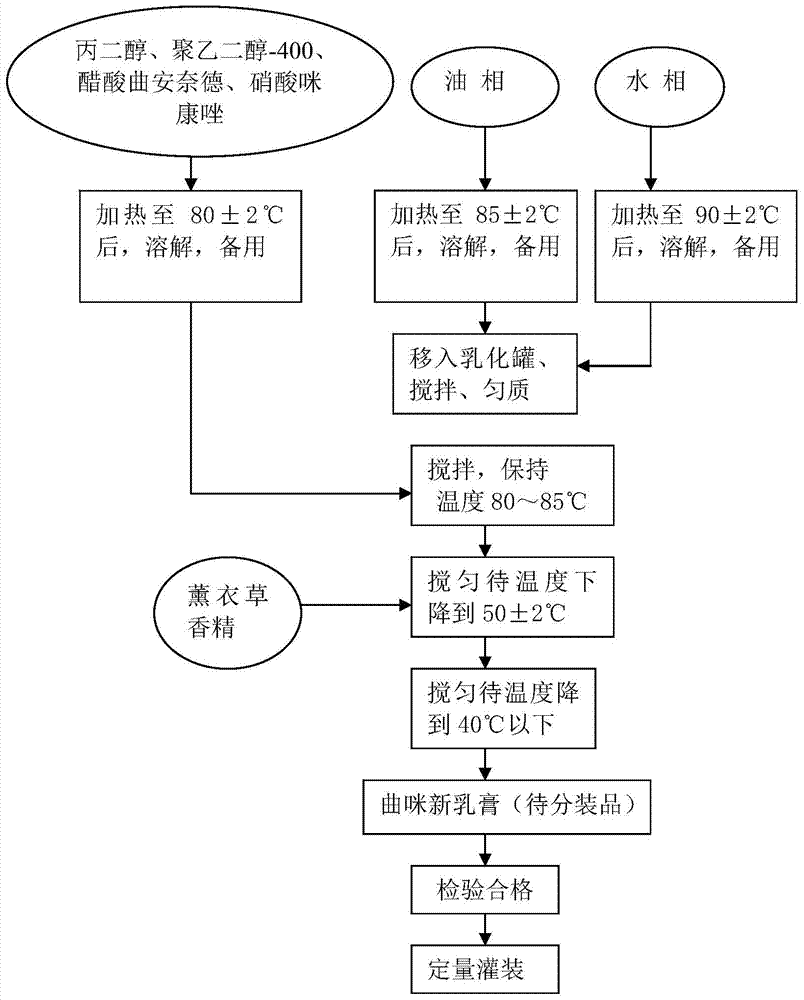
Triamcinolone acetonide acetat and preparation method thereof
ActiveCN103933051AGuaranteed stabilityQuality improvementAntibacterial agentsOrganic active ingredientsDrugs solutionOil phase
The invention relates to triamcinolone acetonide acetat and a preparation method thereof. The preparation method of the triamcinolone acetonide acetat disclosed by the invention comprises the following steps: preparing each batch of raw materials according to the prescription; preparing a main drug solution; preparing an aqueous solution; preparing an oil-phase solution; preparing triamcinolone acetonide acetat: pre-heating an emulsification tank to 50-60 DEG C, starting a vacuum pump, rapidly filtering an oil-phase solution, and sucking into the emulsification tank; starting a stirrer, rapidly sucking the filtered aqueous solution, keeping initial-emulsification temperature at 80-90 DEG C, and homogenizing for 5 minutes; sucking a main drug solution, keeping emulsification temperature at 80-85 DEG C, homogenizing for 8 minutes, stirring, emulsifying, cooling, adding lavender essence, after cooling to be below 40 DEG C, stopping, discharging to obtain triamcinolone acetonide acetat to be sub-packaged, and filling and sealing according to standards. The triamcinolone acetonide acetat prepared through the preparation method disclosed by the invention is high in stability, long in guarantee period, steady and uniform in quality, high in skin penetrability and more rapid in anti-inflammatory, antiallergic and itching-relieving effects; main drug components are uniformly distributed in the triamcinolone acetonide acetat; the preparation method is simple and easy to control, and therefore, the triamcinolone acetonide acetat and the preparation method thereof disclosed by the invention are applied to industrial application.
Owner:TEYI PHARMACEUTICAL GROUP CO LTD
Ocular delivery of triamcinolone acetonide phosphate and related compounds
The present invention provides systems and methods for ocularly delivering a triamcinolone acetonide agent to a subject. In one aspect, for example, a method is provided for treating or preventing an ocular condition in a subject for which triamcinolone acetonide is effective. Such a method may include ocularly administering a triamcinolone acetonide agent to the subject in order to treat or prevent the condition. Although any administration technique is contemplated, in some aspects a triamcinolone acetonide agent may be delivered to eye tissue via ocular iontophoresis.
Owner:ACIONT
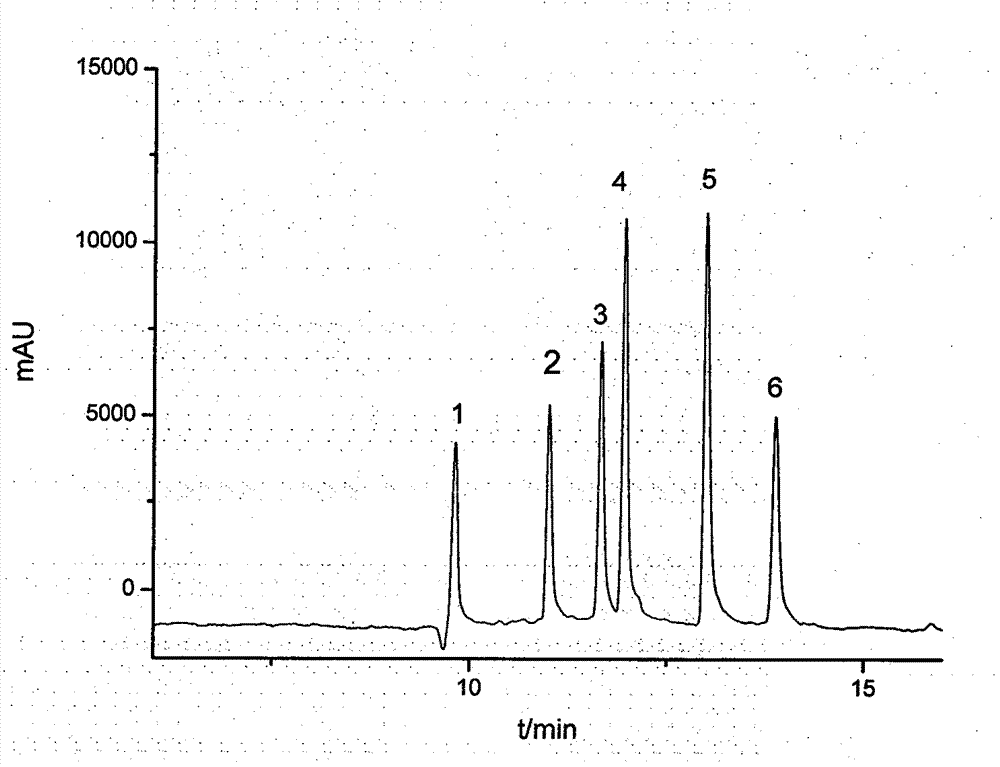
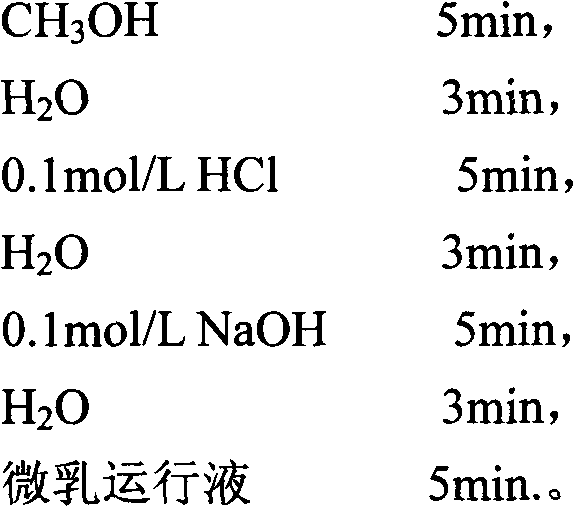
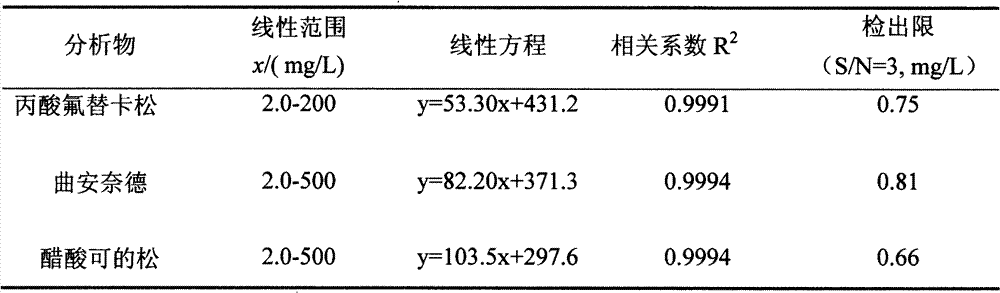
Method for separating and detecting six cortical hormones in skin-care cosmetic
The invention provides a method for separating and detecting six cortical hormones in a skin-care cosmetic. The six cortical hormones, i.e. fluticasone propionate, triamcinolone acetonide, cortisone acetate, dexamethasone, hydrocortisone and prednisone, in the cosmetic are separated and detected by a reverse micro emulsion electrokinetic chromatography (MEEKC) with ionic liquid and beta-cyclodextrin as additives. Compared with a conventional MEEKC separation and detection method, the method has higher separation degree. An experiment result shows that the method is simple in sample treatment, low in using amount, economic, high in speed, and high in separation degree and sensitivity. Capillary electrophoretic detection on the cortical hormones in the cosmetic is realized by the method.
Owner:JIANGNAN UNIV
External use emulsifiable paste for treating skin diseases and with econazole nitrate and triamcinolone acetonide as active components and preparation method
InactiveCN105395562AImprove stabilityUniform contentAntibacterial agentsOrganic active ingredientsDiseaseParaffin wax
The invention discloses external use emulsifiable paste for treating skin diseases and with econazole nitrate and triamcinolone acetonide as active components and a preparation method. The triamcinolone acetonide and econazole emulsifiable paste is prepared from, by weight, 10 parts of econazole nitrate, 1 part of triamcinolone acetonide, 3-10 parts of glycerin monostearate and glycerol distearate, 10-20 parts of stearic acid, 1-10 parts of albolene, 0.8-7 parts of light liquid paraffin, 8-17 parts of glycerinum, 1-10 parts of triethanolamine, 0.08-0.5 part of ethylparaben, 0.08-0.5 part of borax, 0.5-5 parts of dimethyl sulfoxide and 10-60 parts of distilled water. The preparation method includes the steps of oil phase preparation, water phase preparation and finished product preparation. The preparation method includes the steps of oil phase preparation, water phase preparation and finished product preparation. The paste has the advantages that by means of the optimized formula, stability of the preparation is improved, the problem that econazole nitrate and triamcinolone acetonide are difficult to dissolve is solved, and the complex skin diseases caused by fungus and bacterial infection are effectively treated.
Owner:吉林修正药业新药开发有限公司
Triamcinolone acetonide nasal spray with thixotropy and preparation method thereof
ActiveCN106074387ALow viscosityHigh viscosityOrganic active ingredientsAerosol deliveryPreservativeNasal spray
The invention relates to a triamcinolone acetonide nasal spray with thixotropy and a preparation method thereof. The triamcinolone acetonide nasal spray is prepared from, by weight, 0.01%-0.1% of triamcinolone acetonide, 0.5%-2.0% of a compound suspending aid, 0.005%-0.01% of a wetting agent, 4%-6% of an osmotic pressure regulator, 0.010%-0.025% of a preservative, 0.001%-0.05% of a complexing agent, a pH regulating agent enabling the system pH to be kept at 4.5 to 5.5 and deionized water. According to the triamcinolone acetonide nasal spray with the thixotropy and the preparation method thereof, the technological process is simple, convenient to operate and reliable, the prepared nasal spray has the thixotropy, the uniformity of the drugs in the suspension preparation is good, and the nasal spray has the good spreadability when the nasal spray is used and is used for treating allergic rhinitis.
Owner:LIAONING UNIVERSITY
Refining process of triamcinolone acetonide raw medicine
The invention relates to a technology for refining a raw material drug of Triamcinolone. The method comprises the following steps that refining is carried out through recrystallization with a mixed organic solvent, wherein the raw Triamcinolone is added into the mixed solvent of chloroform and carbinol, heated to 50 to 70 DEG C, then a circumfluence under mixing is carried out, and the refined product undergoes the procedures until the content of Triamcinolone exceeds 96 percent, then the refined product undergoes heating circumfluence in ethanol for 2-3 hours, at a temperature of between 70 and 90 DEG C, is cooled down to a temperature below 0 DEG C, filtered by centrifuge, and then dried. The obtained final product is proved completely qualified according to China Pharmacopoeia. The invention can overcome the disadvantages of the prior refining art, and the final product produced by the method has the advantages of stable quality, high content, low impurities, and high yielding rate.
Owner:天津太平洋化学制药有限公司
Triamcinolone acetonide and miconazole emulsion type gel
ActiveCN103142622AGood lookingNo greasy feelingAntibacterial agentsOrganic active ingredientsActive agentChemical compound
The invention discloses triamcinolone acetonide and miconazole emulsion type gel. The gel comprises econazole nitrate and triamcinolone with effective doses, as well as 1 to 9 weight percent of lipophilic phase and 90 to 98 weight percent of hydrophilic phase, wherein the lipophilic phase comprises a lipophilic compound and / or a lipophilic surfactant, and the hydrophilic phase comprises hydrophilic gel matrix, a hydrophilic compound and a hydrophilic surfactant. According to the triamcinolone acetonide and miconazole emulsion type gel prepared by the prescription disclosed by the invention, econazole nitrate and triamcinolone have good in stability and cannot be easily separated out, and the product has a good transparent appearance; due to the low content of lipophilic phase in the product, oily feeling does not exist after the gel is applied to skin; and the hydrophilic gel matrix is contained in the product, so that a transparent film can be formed after the gel is applied to skin, the texture is uniform and smooth, and the gel is easily applied, is not irritant and can be used for increasing the compliance of a patient.
Owner:TAIJI GRP SICHUAN TIANCHENG PHARMA
Compound econazole nitrate film forming gel composition and its use
InactiveCN1899617AGood flexibilitySmall molecular weightOrganic active ingredientsAntimycoticsCellulosePolyol
The compound econazole nitrate film forming gel composition consists of hydroxyalkyl cellulose 0.5-7 wt%, esterifying agent 1-10 wt%, crosslink agent 0.5- 5 wt%, reinforcer 0-5 wt%, solvent 75-90 wt%, econazole nitrate 0.1-5 wt% and triamcinoline aceonide 0.01-3 wt%. The crosslink agent is saturated fatty polyol or alcohol acid in the general expression of CnH2n+2-m-l(OH)m(COOH)l, where, n, m, l are integrals, m is n-2, l is not smaller than 0, m+l is 4-8, and n+l is 4-8. The compound econazole nitrate film forming gel composition is used in treating local fungal inflammation through coating to surface of skin to form one smooth tough antiwear hydrophobic protecting film.
Owner:SHANGHAI INST OF PHARMA IND
Triamcinolone acetonide microsphere preparation and preparation method thereof
ActiveCN109700770AImprove efficiencyHigh yieldOrganic active ingredientsSkeletal disorderRelease modulatorMicrosphere
The invention provides a preparation method of a triamcinolone acetonide microsphere preparation. The method comprises the steps of: (1) dissolving PLGA in a volatile and well-soluble organic solventso as to form a homogeneous-phase solution; (2) adding triamcinolone acetonide to the homogeneous-phase solution described in the step (1) to be dissolved to obtain a homogeneous-phase solution with afinal viscosity of 5-500cp; and (3) feeding the homogeneous-phase solution described in the step (2) to a cup-shaped container in the center of a turntable device, wherein the liquid in the cup-shaped container passes over a cup rim, the solution hits a lateral disc-shaped turntable under the action of centrifugal force and gravity so as to form microdroplets, the formed microdroplets continue tohit a more lateral disc-shaped turntable, and after two or more hitting, the microdroplets fly out of the turntable and are solidified to form microspheres. The microsphere preparation obtained by the method has outstanding sustained release ability, and requires no additional release regulator, and the sustained release period can be as long as 1-3 months.
Owner:ZHEJIANG SUNDOC PHARMA SCI & TECH CO LTD
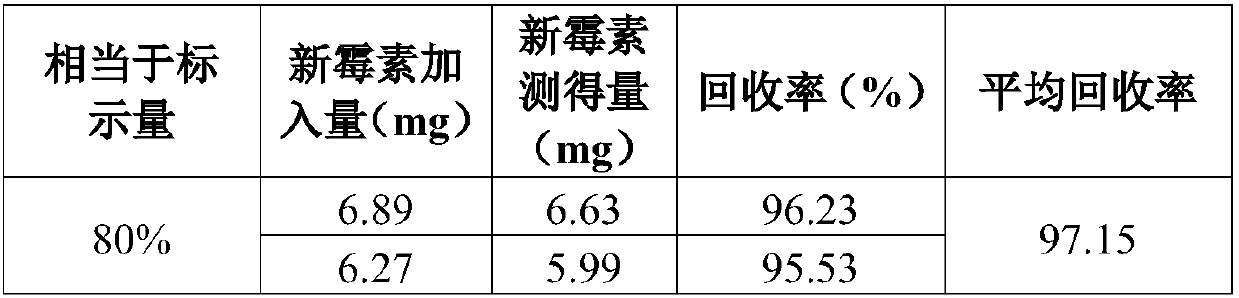
Method for detecting neomycin sulfate content in triamcinolone acetonide acetat
InactiveCN107858397AStrong specificityGood repeatabilityMicrobiological testing/measurementBiological material analysisSolubilityNeomycin Sulfate
The invention provides a method for detecting detecting neomycin sulfate content in triamcinolone acetonide acetat. The method comprises the following steps: preparing bacterium suspension; preparinga standard solution; preparing a test solution; preparing a negative sample solution; culturing; detecting. The method is high in specificity and repeatability; compared with a liquid chromatography,the method has the advantages of being simple to operate, low in test cost, and convenient to popularize and apply; dichloromethane is treated as a solvent, so that the solubleness is high, and creamsubstrate can be quickly dissolved in dichloromethane; the influence of the cream substrate is greatly avoided; the detecting neomycin sulfate content detecting result is high in reliability and highin accuracy degree.
Owner:广西壮族自治区食品药品检验所
Low viscosity, highly flocculated triamcinolone acetonide suspensions for intravitreal injection
ActiveUS20090233890A1Improved stability characteristicsEasily resuspendedPowder deliveryOrganic active ingredientsViscosityBiomedical engineering
Triamcinolone acetonide suspension compositions are disclosed. The suspension compositions have a relatively low viscosity and are easy to extrude through a 27- or 30-gauge needle but are highly flocculated and easily redispersed. The compositions are particularly suitable for intravitreal injection.
Owner:NOVARTIS AG
Sterically stabilized liposome and triamcinolone composition for treating the respiratory tract of a mammal
InactiveUS20060115523A1Extend effective lifeLiposomal deliveryEffective treatmentAerosol drug delivery
This invention relates to a composition containing a sterically stabilized liposome and triamcinolone, effective for the treatment of a mammal, with the composition being adapted for administration as an aerosol and with the composition providing effective treatment for a period of time at least 1.5 times as long as the effective time for treatment with triamcinolone alone. This invention also relates to a method for treating a mammal respiratory tract with the composition.
Owner:VGSK TECH
High efficiency and low toxicity externally applied medicine preparation of ginsenoside and triamcinolone acetonide
The present invention is externally applied medicine composition comprising glucocorticoid hormone and ginsenoside and with high curative effect and less side effect. Glucocorticoid hormone is important medicine for treating dermatosis but has some serious untoward reactions. It is found that the composition of ginsenoside and triamcinolone acetonide as one glucocorticoid hormone has strengthened anti-inflammatory action and less untoward reactions, and may be used in treating various kinds of dermatosis.
Owner:GUANGDONG MEDICAL UNIV
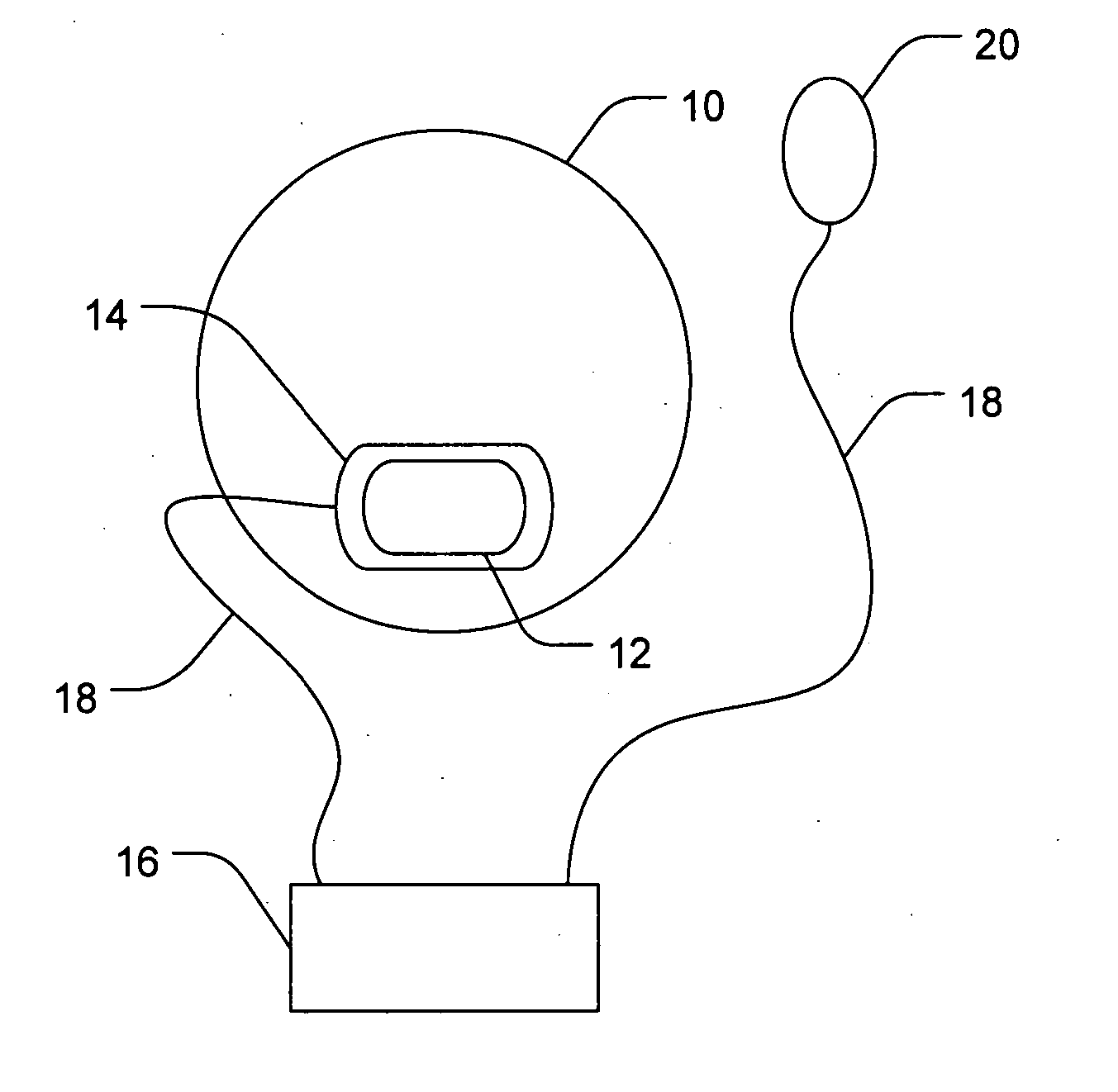
Medical Kits
Provided is a medical kit with all the necessary components for a medical practitioner in the office practice setting to inject a variety of anesthetics. The kits can comprise a holder, such as a box, and active pharmaceutical ingredients (API), with each API placed in a separate container in a form that is ready for injection, the API selected from the group consisting of: a. lidocaine hydrochloride and triamcinolone acetonide with or without a separate container of ammonia; b. bupivacaine hydrochloride (HCl) and lidocaine hydrochloride (HCl), and optionally one or more of triamcinolone acetonide, methylprednisolone acetate, or dexamethasone sodium; c. bupivacaine hydrochloride (HCl) and one or more of triamcinolone acetonide or methylprednisolone acetate; d. Methylprednnisolone acetate and lidocaine hydrochloride (HCl), and optionally bupivacaine hydrochloride (HCl); e. methylprednisolone acetate and lidocaine hydrochloride (HCl), and bupivacaine hydrochloride (HCl); f. lidocaine hydrochloride, triamcinolone acetonide, and ammonia; g. bupivacaine hydrochloride or lidocaine HCl, and betamethasone sodium phosphate and betamethasone acetate; h. bupivacaine hydrochloride or lidocaine HCl, and dexamethasone sodium phosphate; i. ketorolac tromethamine, lidocaine HCl, and optionally bupivacaine hydrochloride; and j. one or more of dexamethasone sodium.
Owner:ASCLEMED USA
Combination therapy comprising a thiazole and a corticosteroid to treat skin conditions
InactiveUS20190255023A1Preferable effectReduce inflammation and itchinessOrganic active ingredientsAerosol deliveryFluocinoloneFluocinonide
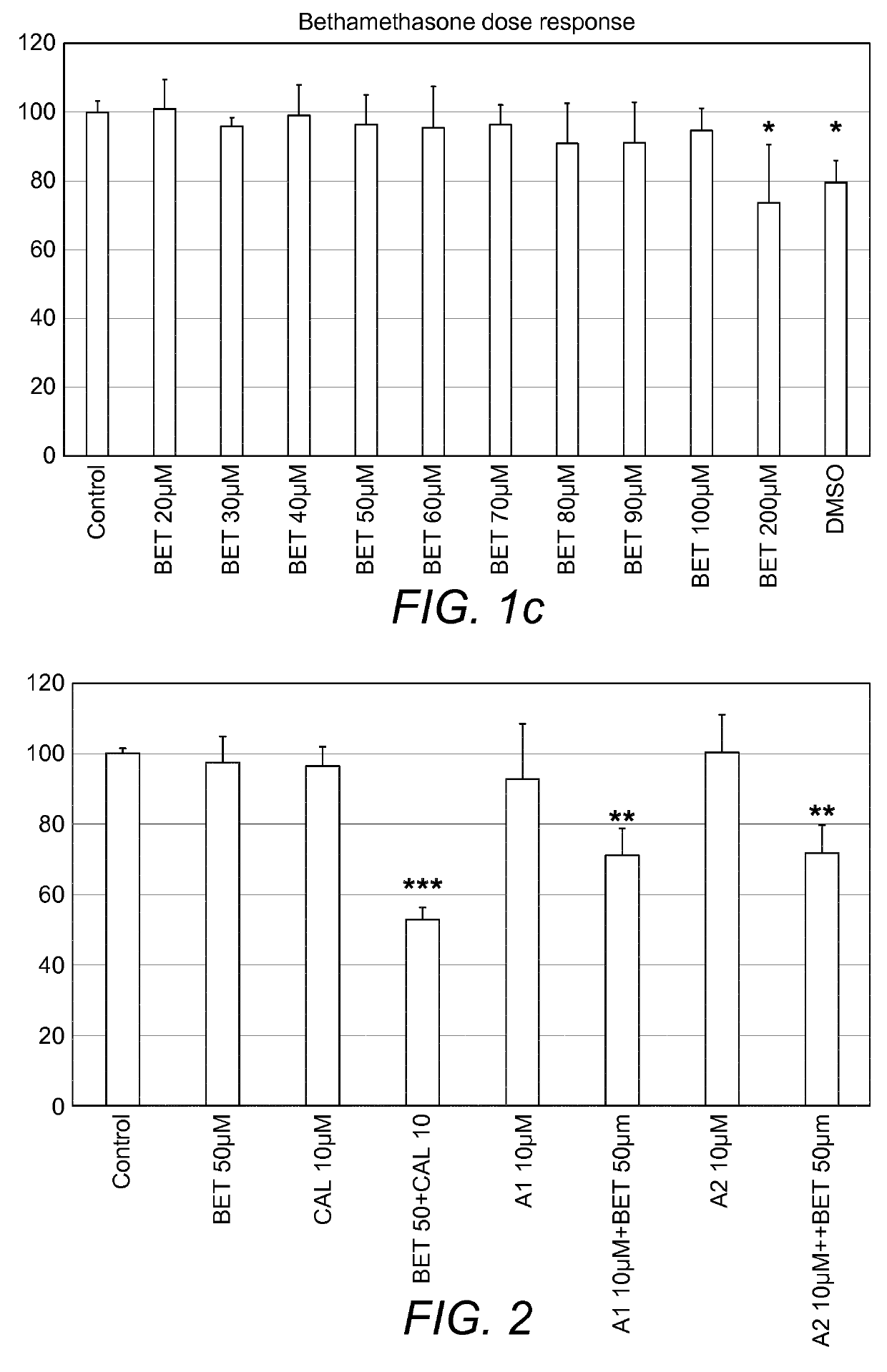
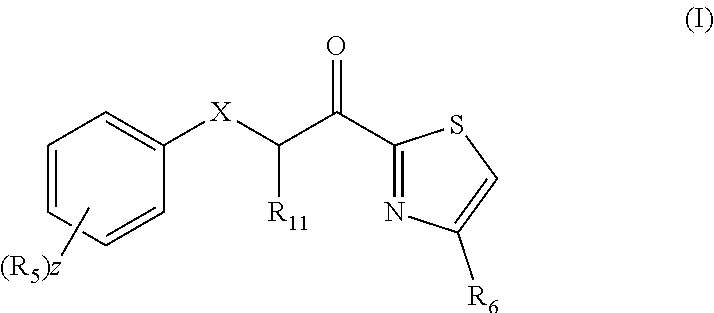
A pharmaceutical composition comprising: (A) at least one compound of formula (I): wherein X is O or S, preferably O R6 is H, C1-6alkyl, —(CH2)pCOOH, —(CH2)pCOOC1-6alkyl, —(CH2)pCONH2, —(CH2)pCONHC1-6alkyl, —(CH2)pCON(C1-6alkyl)2, R11 is H or C1-6 alkyl; each R5 is —OC1-10alkyl, —SC1-10alkyl, —C1-12alkyl, or OAr2; wherein Ar2 is phenyl, optionally substituted with one or more halo; each p is 0 to 3; each z is 1 to 2; or a pharmaceutically acceptable salt, or a hydrate or solvate thereof; and (B) one or more corticosteroid partners, preferably selected from the group consisting of betamethasone, clobetasol, halometasone, dexamethasone, fluocortolone, desoximetasone, diflorasone, fluocinonide, flurandrenolide, halobetasol, amcinonide, halocinonide, triamcinolone, hydrocortisone, aclometasone, fluticasone, mometasone, clocortolone, fluocinolone, desonide, prednisone, prednisolone, and prednicarbate or a pharmaceutically acceptable salt, or a hydrate or solvate thereof, especially betamethasone or a pharmaceutically acceptable salt, or a hydrate or solvate thereof.
Owner:AVEXXIN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com