Refining process of triamcinolone acetonide raw medicine
A raw material drug and process technology, applied in the technical field of triamcinolone acetonide raw material drug refining, to achieve the effect of high content, high yield and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
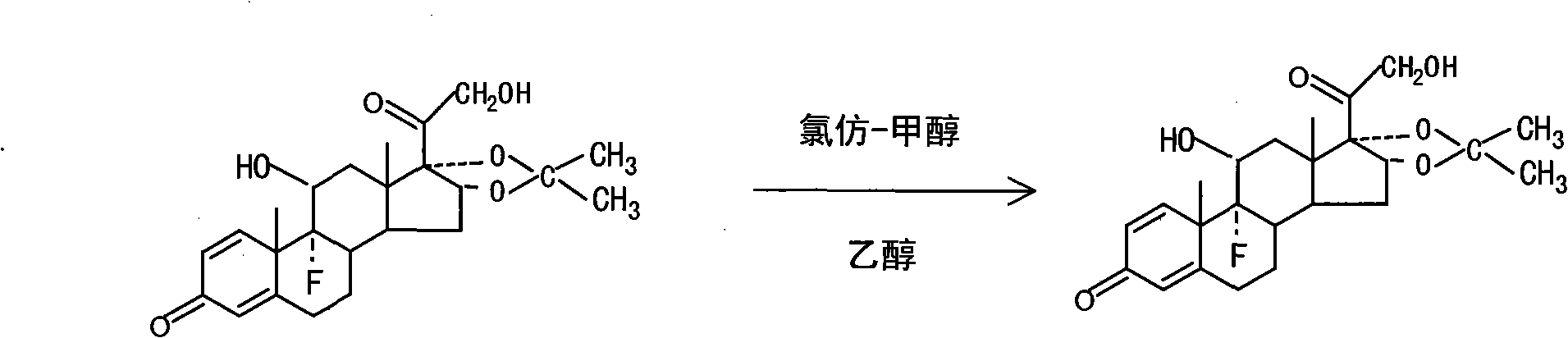
[0018] Embodiment 1: a kind of process of triamcinolone acetonide crude drug refining, adopts mixed organic solvent recrystallization to refine, and it comprises the following steps:
[0019] 1), at 30°C, put 30kg of triamcinolone acetonide crude product into 300kg of chloroform and 300kg of methanol mixed solvent, heat to reflux at 60°C, stir and react for 1.5 hours, after the reaction is completed, cool to below 0°C, shake off and dry to obtain koji Acetonide chloroform-methanol primary refinement 28.5kg, content 95.1%;
[0020] 2), at 20°C, repeat step 1) with 28.5kg of the primary refined product, put it into 285kg of chloroform and 285kg of methanol mixed solvent, heat to 70°C and reflux, stir and react for 2 hours, after the reaction is completed, cool, filter, and dry to obtain Triamcinolone acetonide chloroform-methanol secondary refinement 27.1kg, content 96.2%;
[0021] 3), at 50°C, put 27.1kg of triamcinolone acetonide chloroform-methanol secondary refined product ...
Embodiment 2
[0022] Embodiment 2: a kind of process of triamcinolone acetonide crude drug refining, adopts mixed organic solvent recrystallization to refine, and it comprises the following steps:
[0023] 1), at 30°C, put 30kg of crude triamcinolone acetonide into 700kg of chloroform and 100kg of methanol mixed solvent, heat to reflux at 56°C, stir and react for 1.5 hours. Acetonide chloroform-methanol primary refinement 28.8kg, content 95.0%;
[0024] 2), at 20°C, repeat step 1) with 28.8kg of primary refined product, put it into 672kg of chloroform and 96kg of methanol mixed solvent, heat to 56°C and reflux, stir and react for 2 hours, after the reaction is completed, cool, filter, and dry to obtain Triamcinolone acetonide chloroform-methanol secondary refinement 27.7kg, content 96.0%;
[0025] 3), at 50°C, put 27.7kg of triamcinolone acetonide chloroform-methanol secondary refinement into 1108kg of ethanol with a content of 80%, heat to reflux at 80°C, stir and react for 2.5 hours, aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com