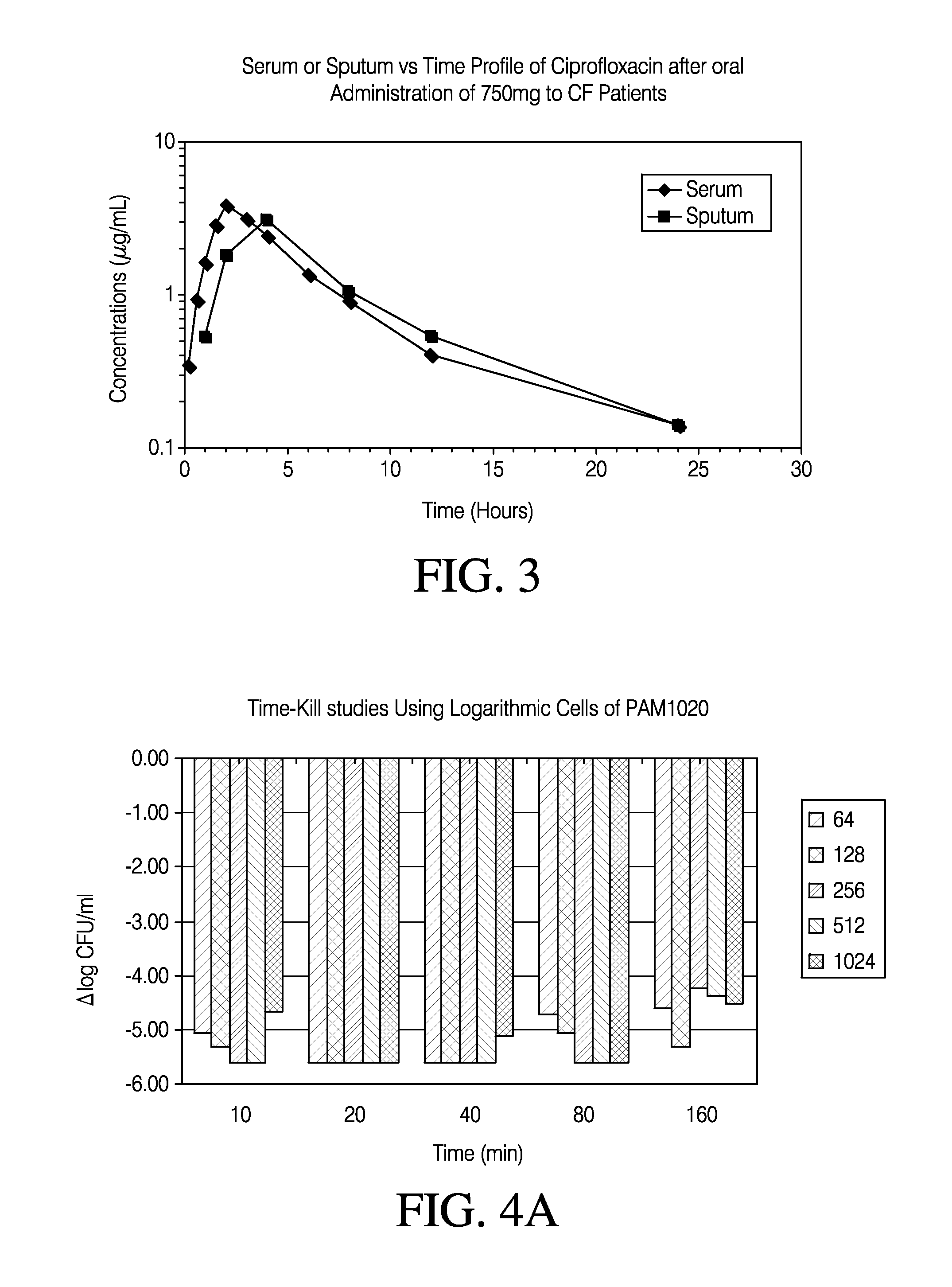
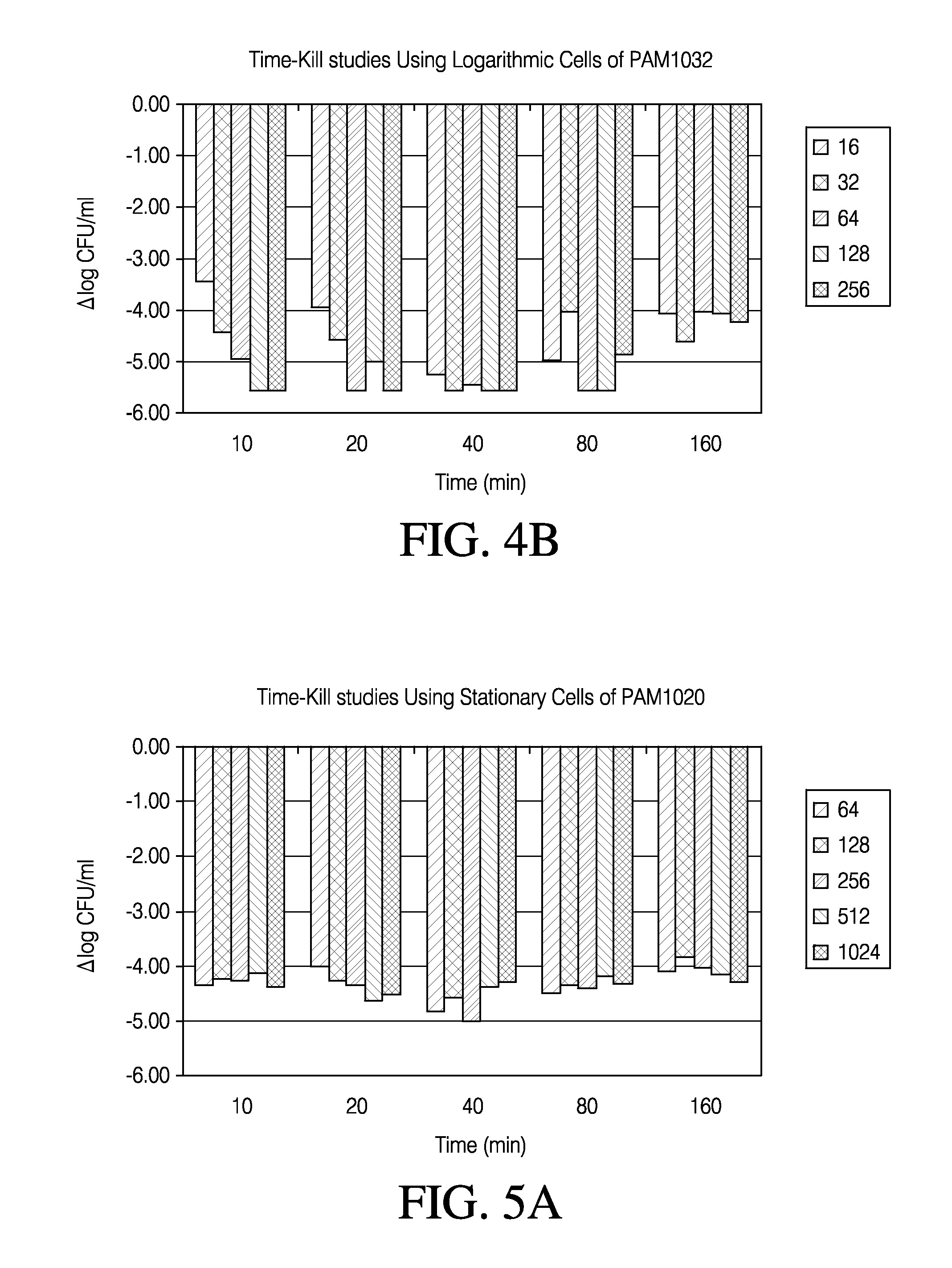
Patents
Literature
54 results about "Aerosol drug delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
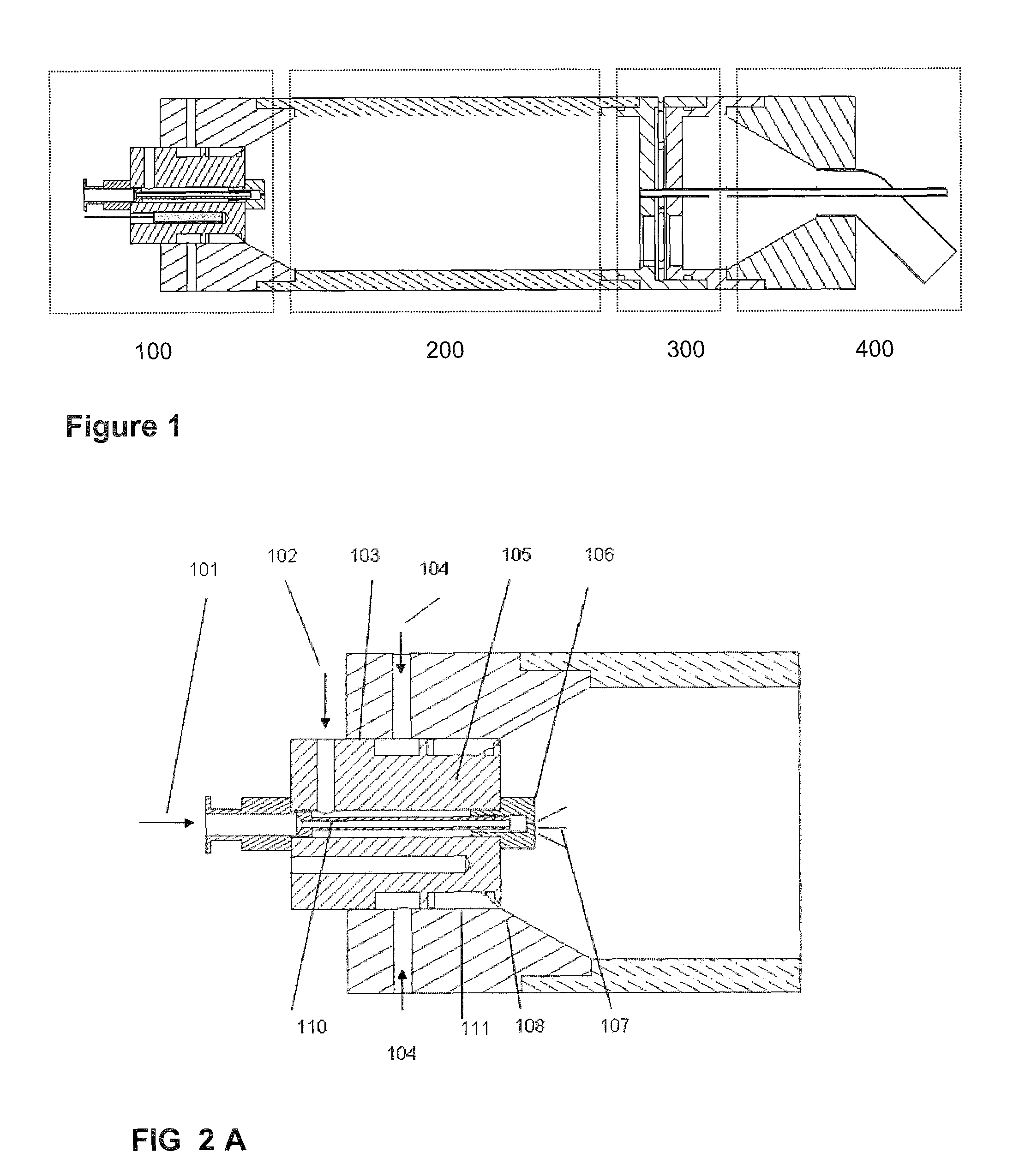
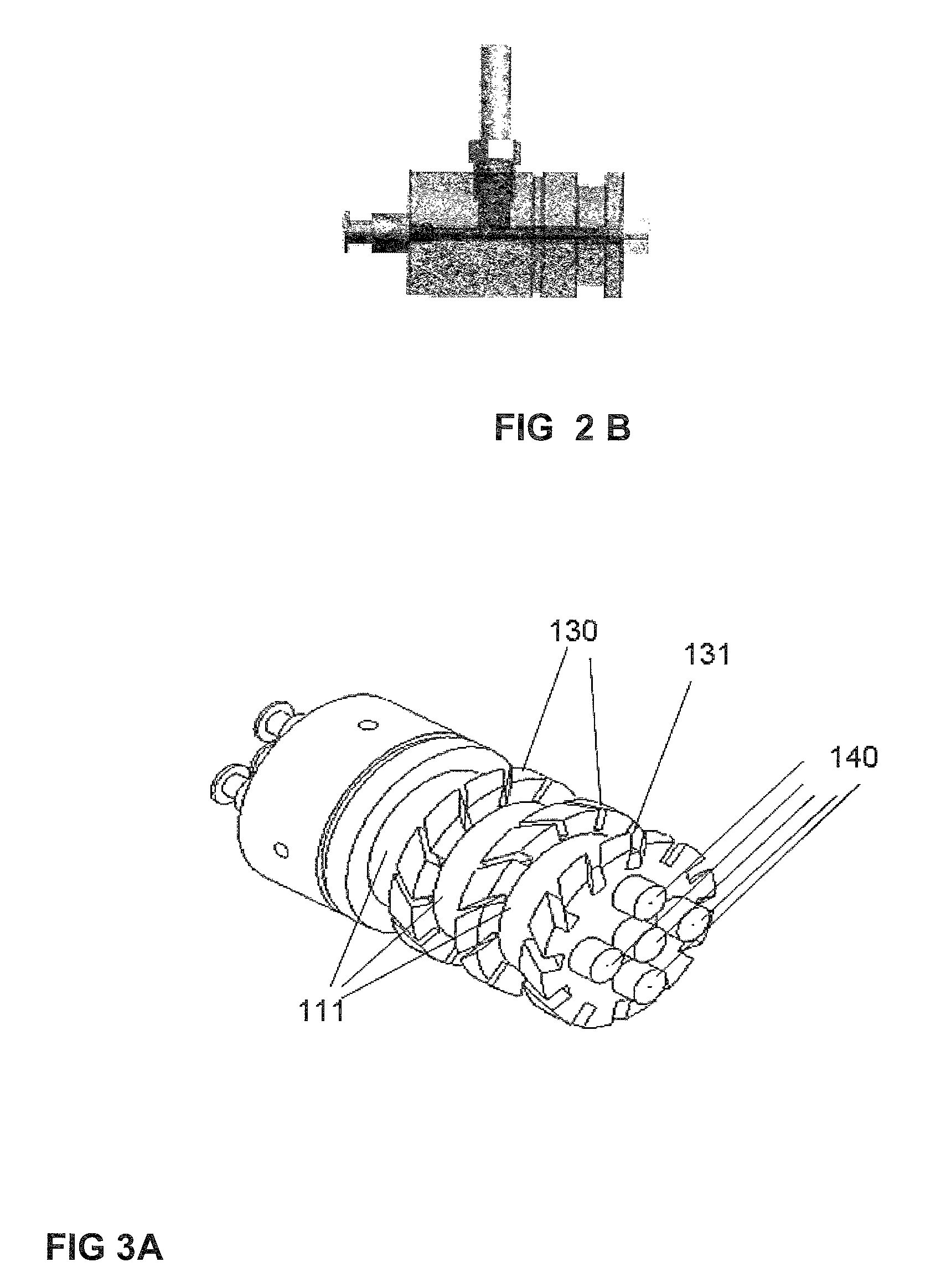
Aerosol processing and inhalation method and system for high dose rate aerosol drug delivery
ActiveUS20070144514A1Increase dose rateRisk minimizationRespiratorsLiquid surface applicatorsSolvent vaporCounter flow
A method and system is disclosed which is capable of delivering at a high dose rate, respirable solid aerosols derived from aqueous- or nonaqueous-based solutions containing the desired therapeutic agent(s). The method and system comprises the integration of an aerosol generator, an aerosol evaporator, an aerosol concentrator, and an aerosol flow regulator. The aerosol generator generates 10-30 μm droplets, with a narrow size distribution. The aerosol jet is arrested by a coaxial counter-flow heated air jet, and evaporated rapidly by annular swirling heated air. Most of the air, together with the unwanted solvent vapor, is removed from the aerosol stream during the process of aerosol concentration. The output aerosol carries the dry particles to be inhaled by the patient. The respiratory-governed control of aerosol fluid generation system delivers fluid containing the test agent of interest (drug or toxin) to the aerosol generator throughout inhalation.
Owner:KAER BIOTHERAPEUTICS CORP
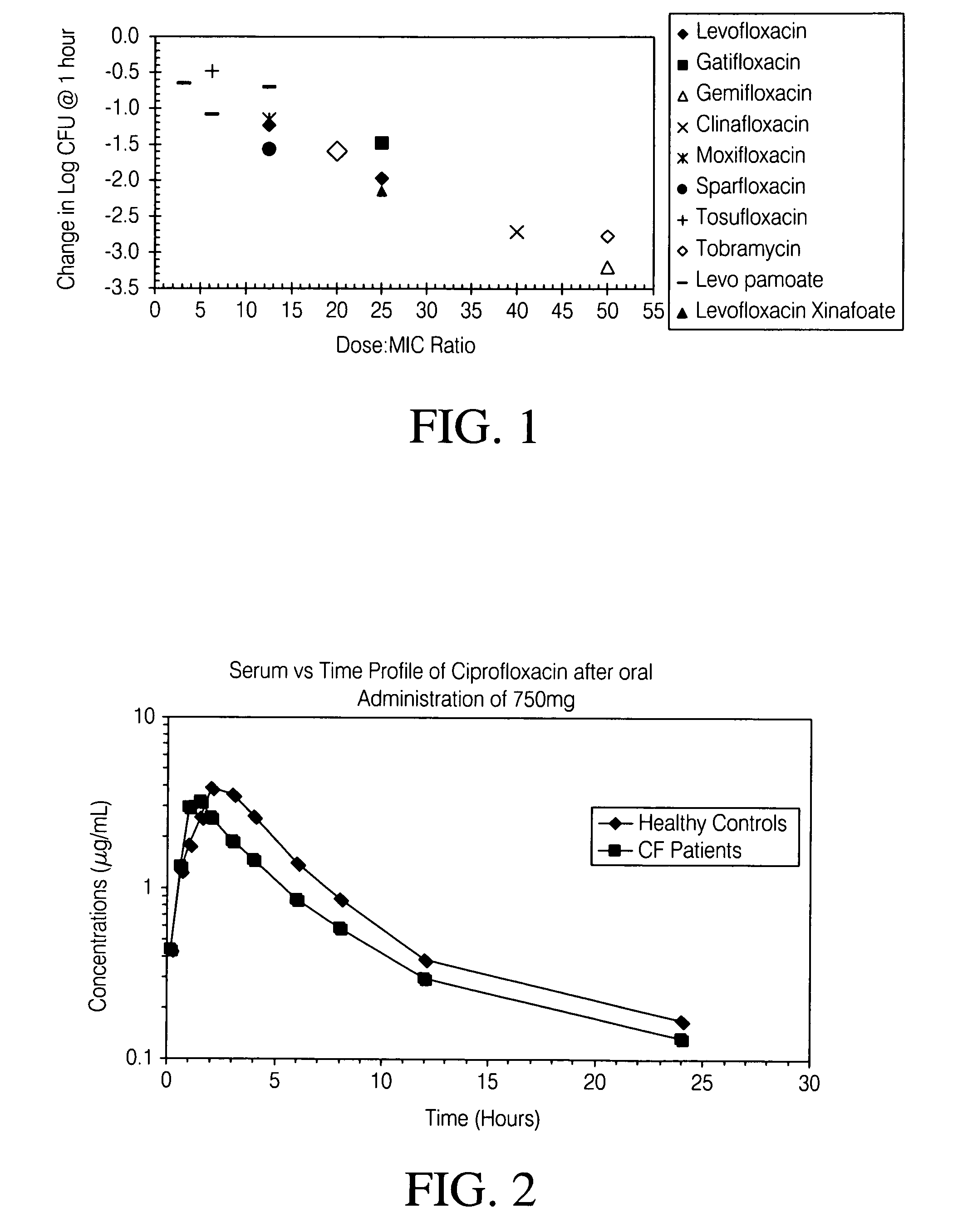
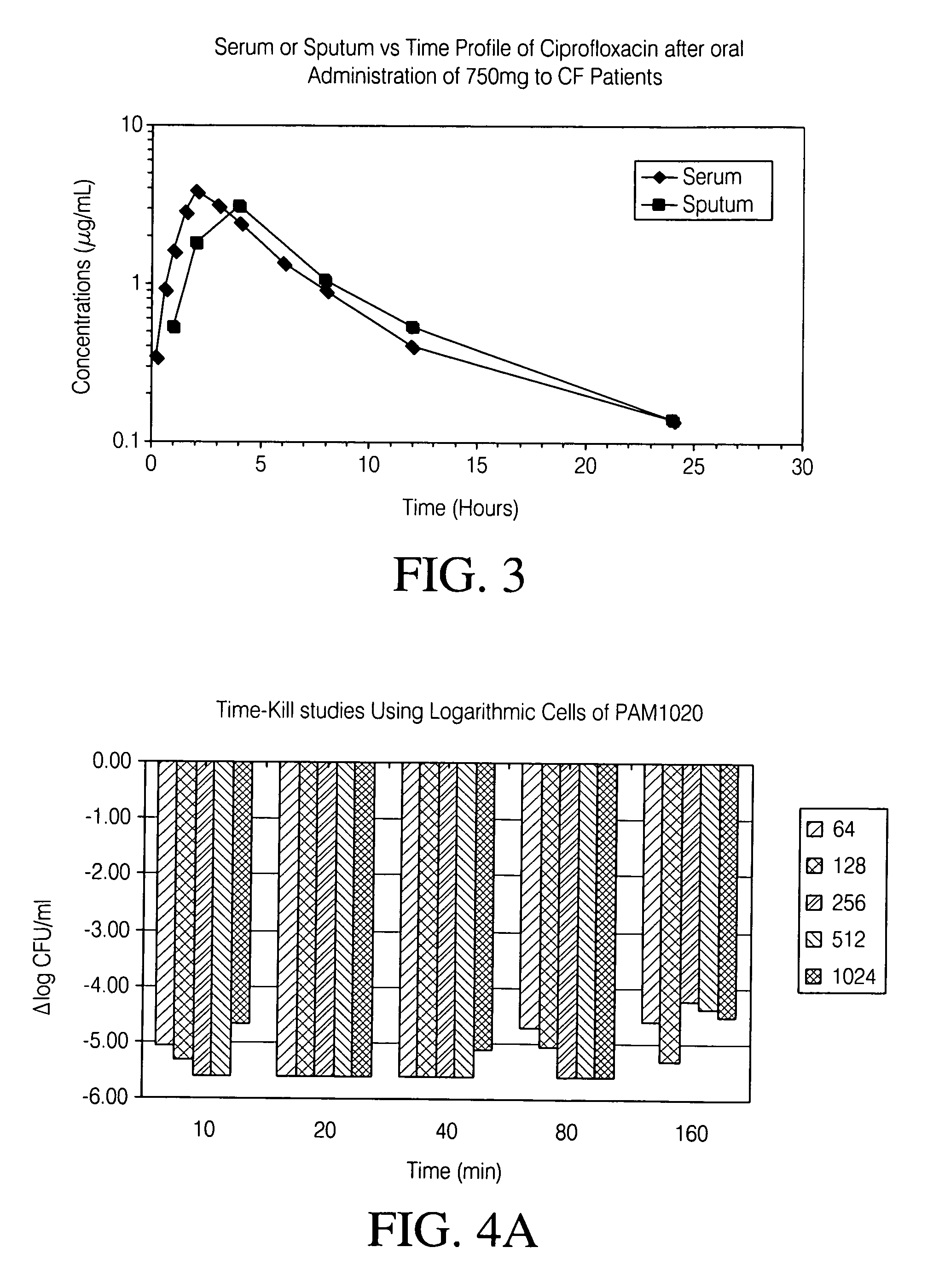
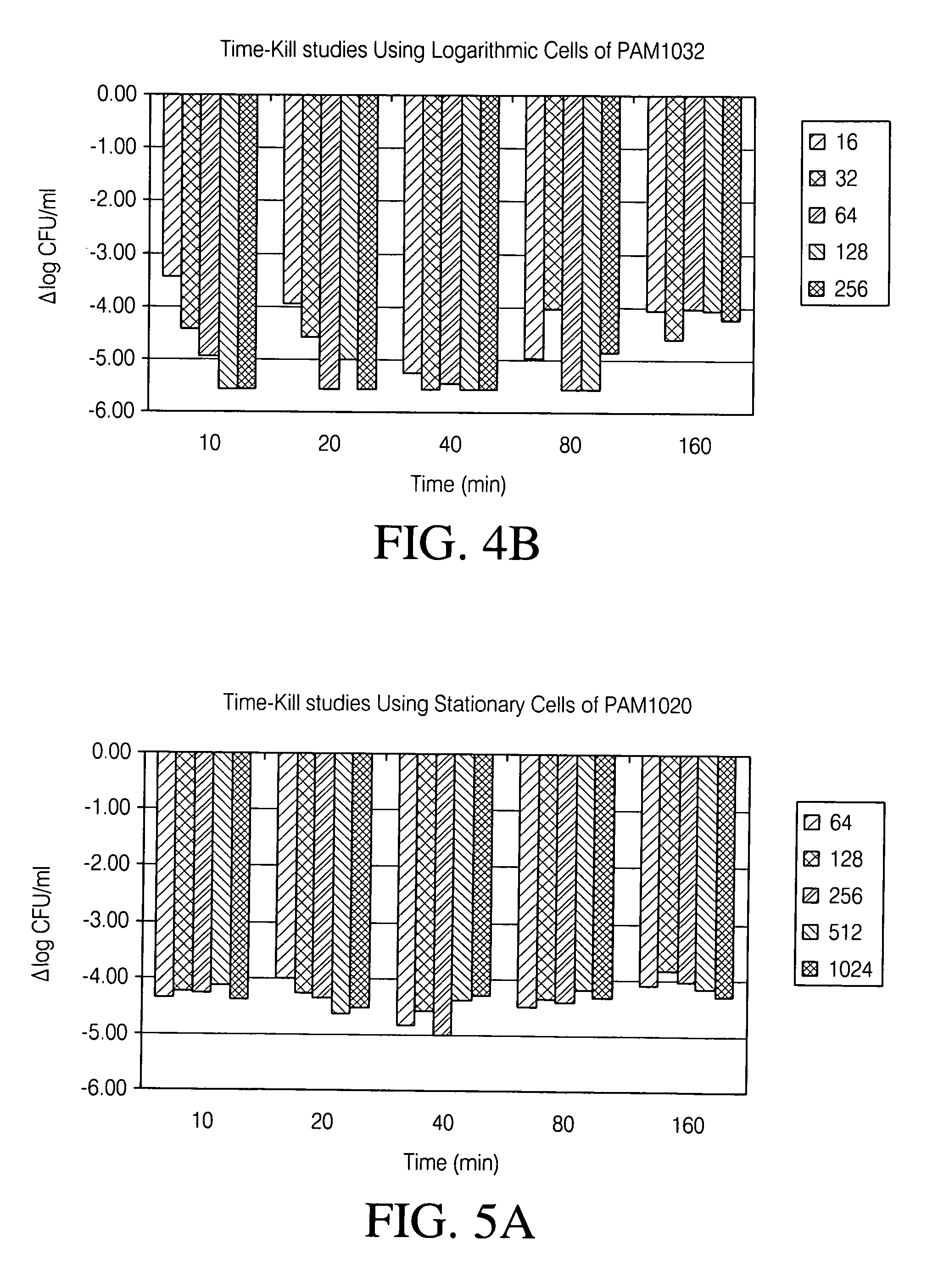
Aerosolized fluoroquinolones and uses thereof
ActiveUS20060276483A1Reduce riskHigh level of drugBiocideHeavy metal active ingredientsAerosol drug deliveryAntimicrobial
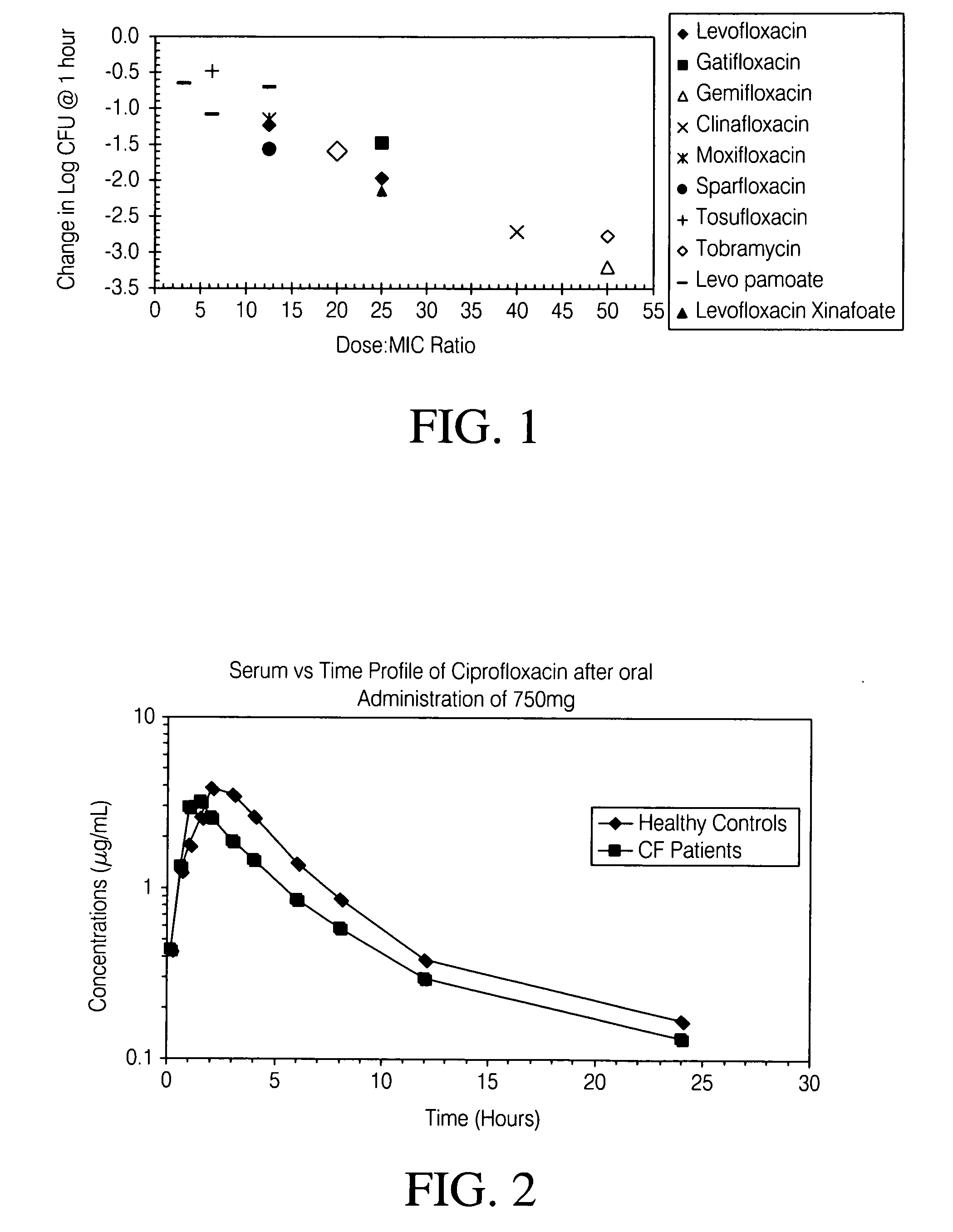
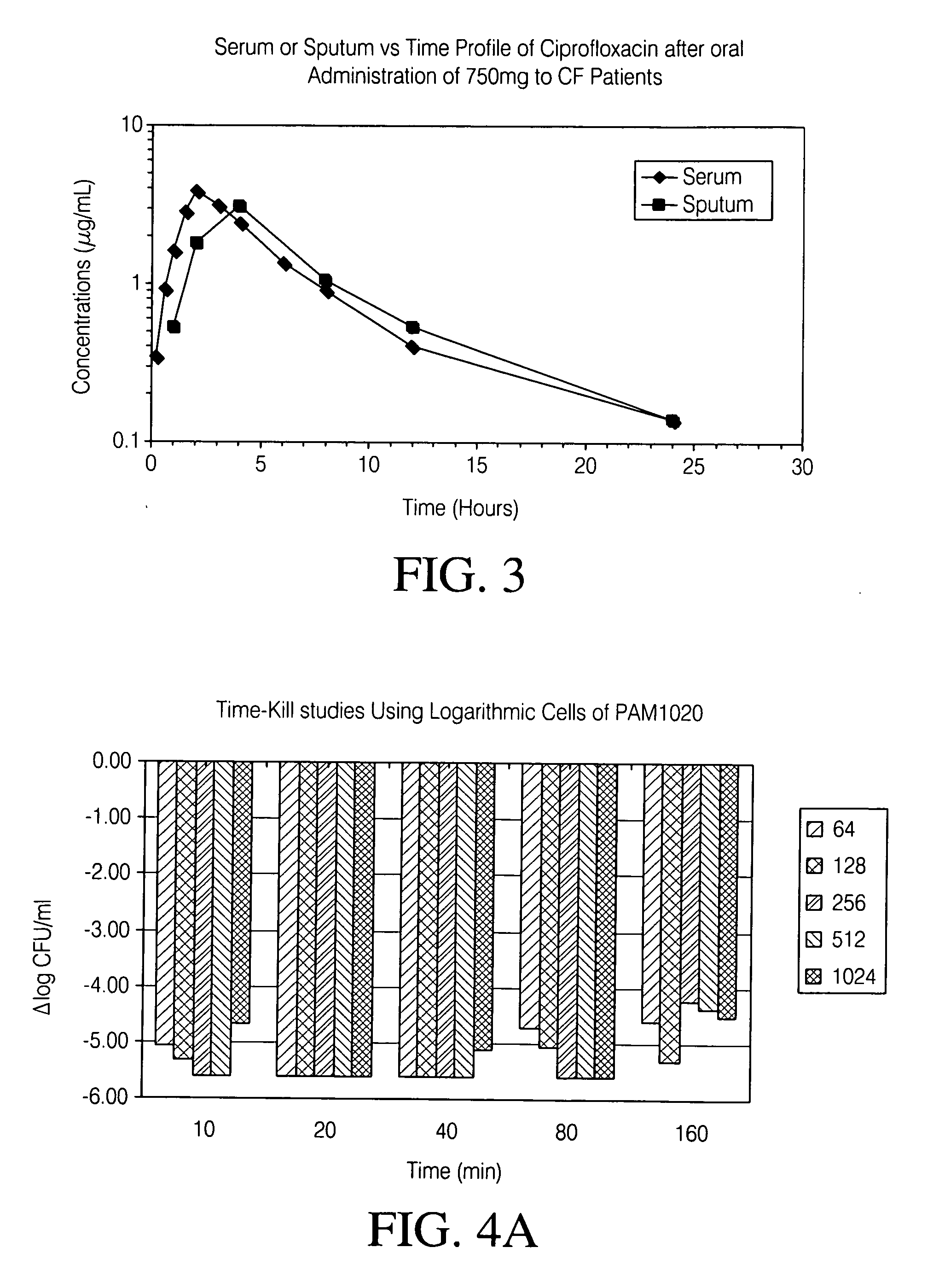
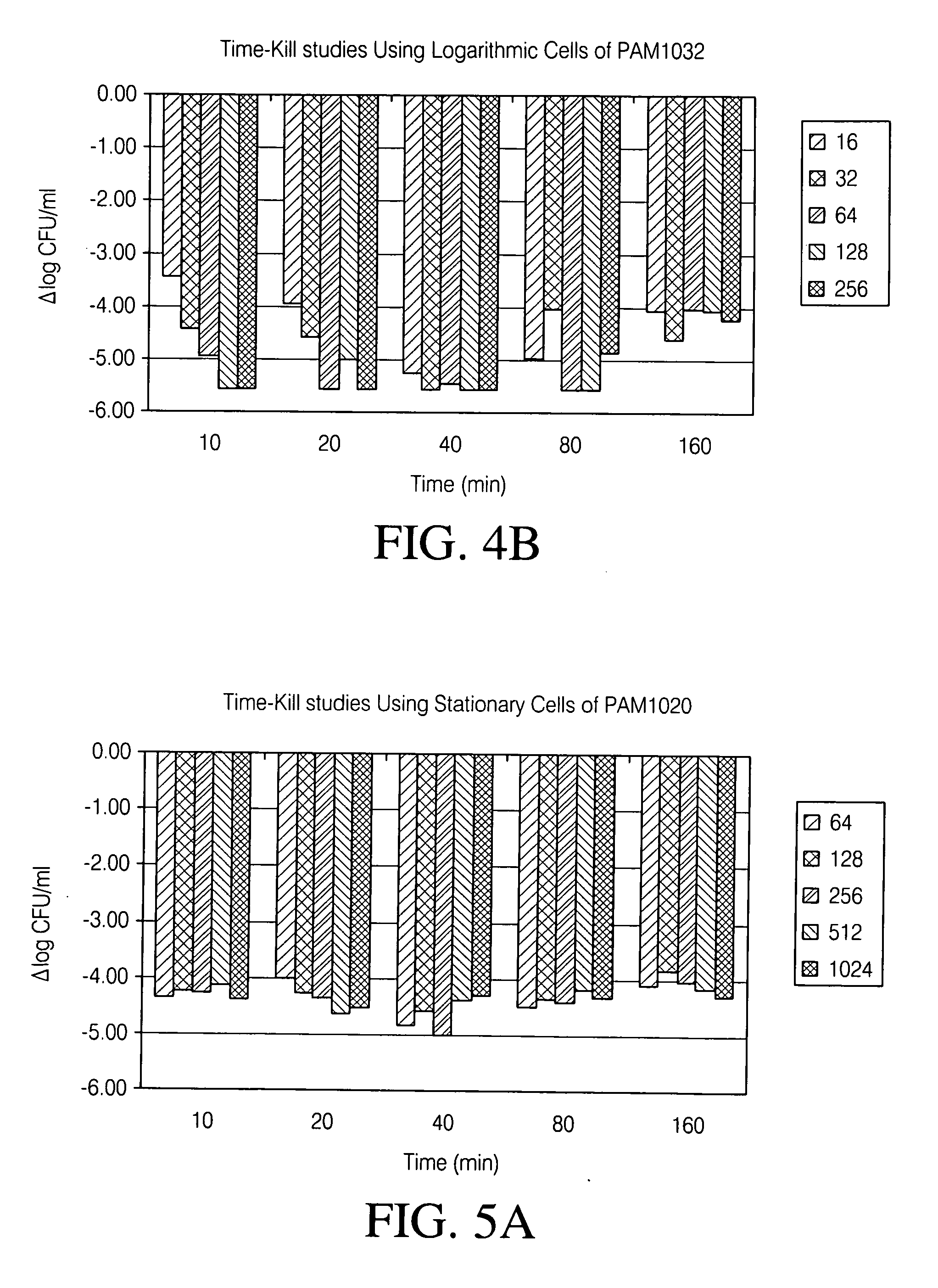
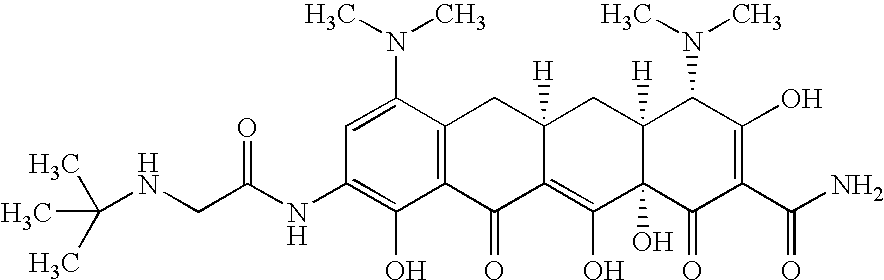
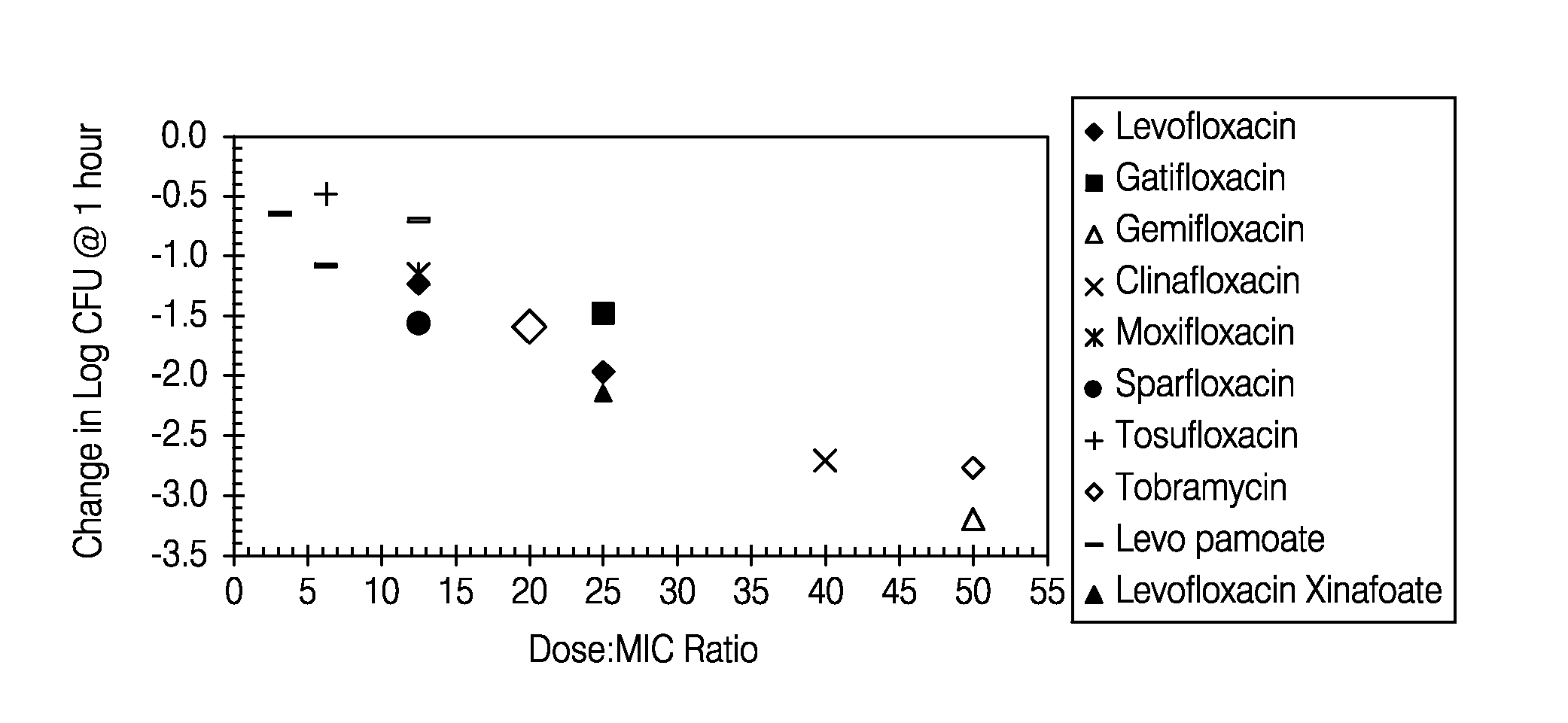
Disclosed herein are formulations of fluoroquinolones suitable for aerosolization and use of such formulations for aerosol administration of fluoroquinolone antimicrobials for the treatment of pulmonary bacterial infections. In particular, inhaled levofloxacin specifically formulated and delivered for bacterial infections of the lungs is described. Methods include inhalation protocols and manufacturing procedures for production and use of the compositions described.
Owner:HORIZON ORPHAN LLC
Aerosol processing and inhalation method and system for high dose rate aerosol drug delivery
ActiveUS7802569B2Risk minimizationIncrease dose rateRespiratorsDispersed particle separationCounter flowSolvent vapor
Owner:KAER BIOTHERAPEUTICS CORP
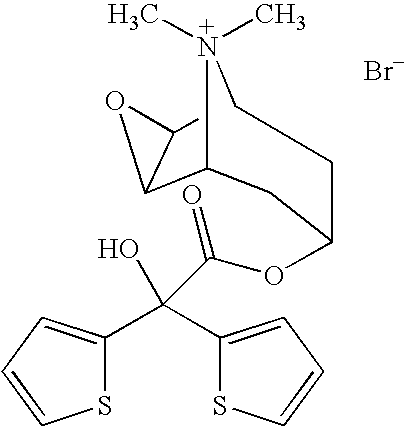
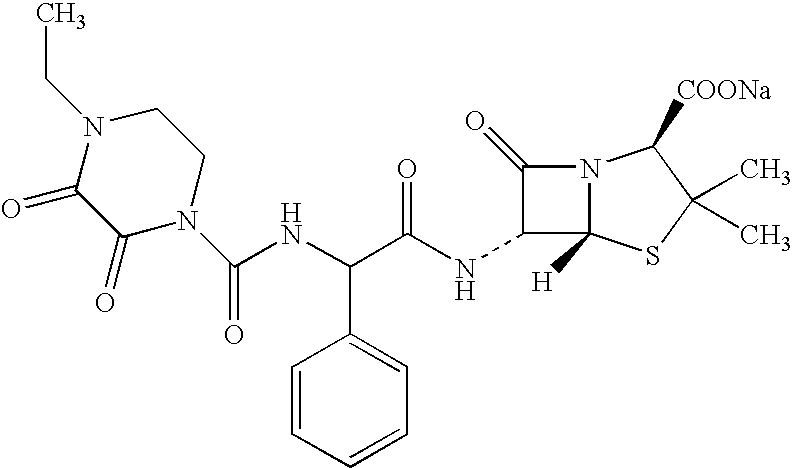
Tiotropium containing HFC solution formulations
This invention relates to tiotropium containing stable pharmaceutical solution formulations suitable for aerosol administration. More particularly, this invention relates to tiotropium containing stable pharmaceutical solution formulations suitable for aerosol administration wherein either an inorganic acid or an organic acid is added to the aerosol solution formulation which contains a tiotropium salt, preferably tiotropium bromide in solution with an environmentally safe hydrofluorocarbon (HFC) as a propellant, together with an organic compound as a cosolvent. The acid provides stability against degradation or decomposition of the medicament resulting largely from interaction of the medicament with the cosolvent and / or water present in the solution formulation.
Owner:BOEHRINGER INGELHEIM PHARM KG
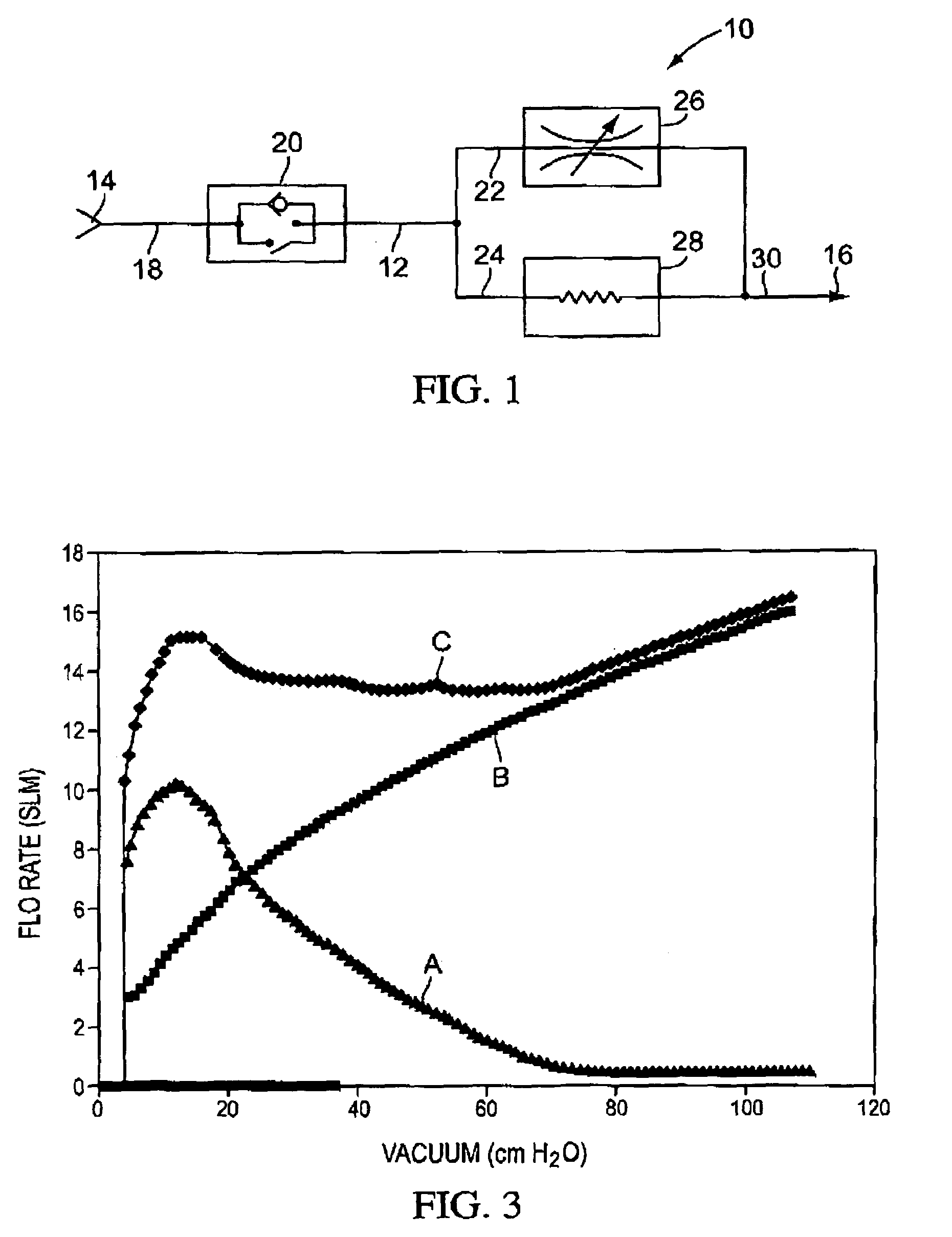
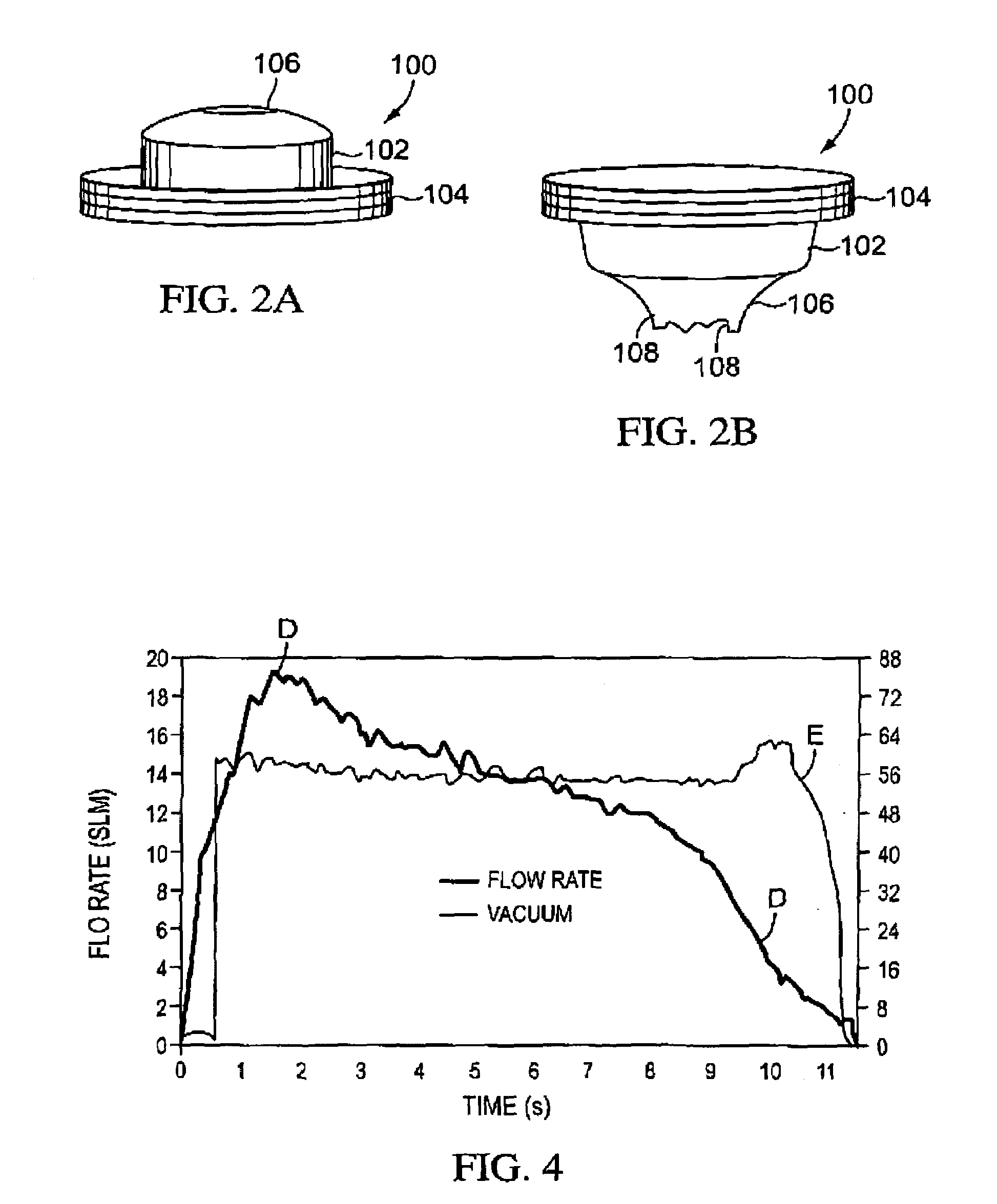
Flow regulator for aerosol drug delivery and methods
InactiveUS7185651B2Sufficient flow rateEasy to useRespiratorsLiquid surface applicatorsVacuum levelEngineering
An aerosolization device comprises a housing having a mouthpiece, and a flow path arrangement in fluid communication with the mouthpiece. The flow path arrangement has a flow regulating valve and a threshold valve, where the threshold valve is configured to open at a first vacuum level and to close at a second vacuum level that is less than the first vacuum level. The housing includes a region that is adapted to hold a powder in fluid communication with the flow path arrangement so that air drawn through the mouthpiece opens the threshold valve once the first vacuum level is exceeded and remains open until the vacuum falls below the second vacuum level. The flow rate of the air drawn through the mouthpiece is regulated by the flow regulating valve to remain within a certain range while the threshold valve remains open.
Owner:NOVARTIS FARMA
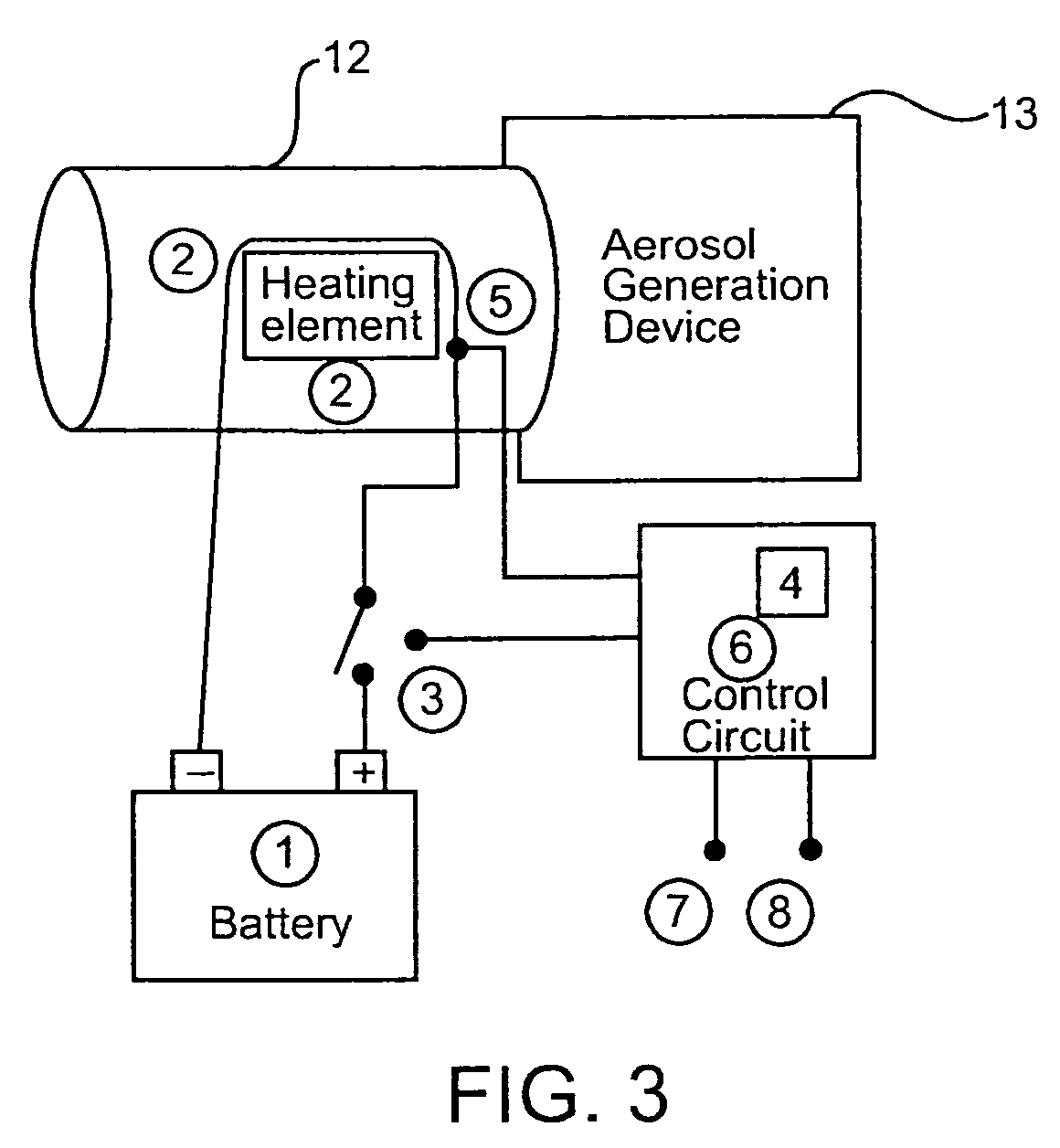
Temperature controlling device for aerosol drug delivery
InactiveUS7143766B2Improve drug delivery efficiencyGood repeatabilityRespiratorsOther heat production devicesAerosol drug deliveryDrug formulations
A portable air temperature controlling device useful for warming air surrounding an aerosolized drug formulation. Warming the air of an aerosol makes it possible to reduce the diameter of aerosol particles produced by an aerosol generation device. Additionally, warming the air forces the diameter of the aerosol particles to be in the range required for systemic drug delivery independent of ambient conditions. Smaller particles can be more precisely targeted to different areas of the respiratory tract.
Owner:ARADIGM
Liquid formulation comprising nicotine for aerosol administration
In one aspect, the present invention features a method of administering nicotine or a salt thereof to a human, wherein the method includes inhaling an aerosol of a liquid formulation, the liquid formulation comprising: (i) at least 12 percent by weight of water; (ii) at least 70 percent by weight of propylene glycol; and (iii) at least 2 percent by weight of said nicotine or a salt thereof; wherein the liquid formulation includes no more than 5 percent by weight of glycerol and no more than 5 percent by weight of ethanol. The present invention also features an aerosol-generating device and a reservoir for such a device containing such a liquid formulation.
Owner:MCNEIL AB
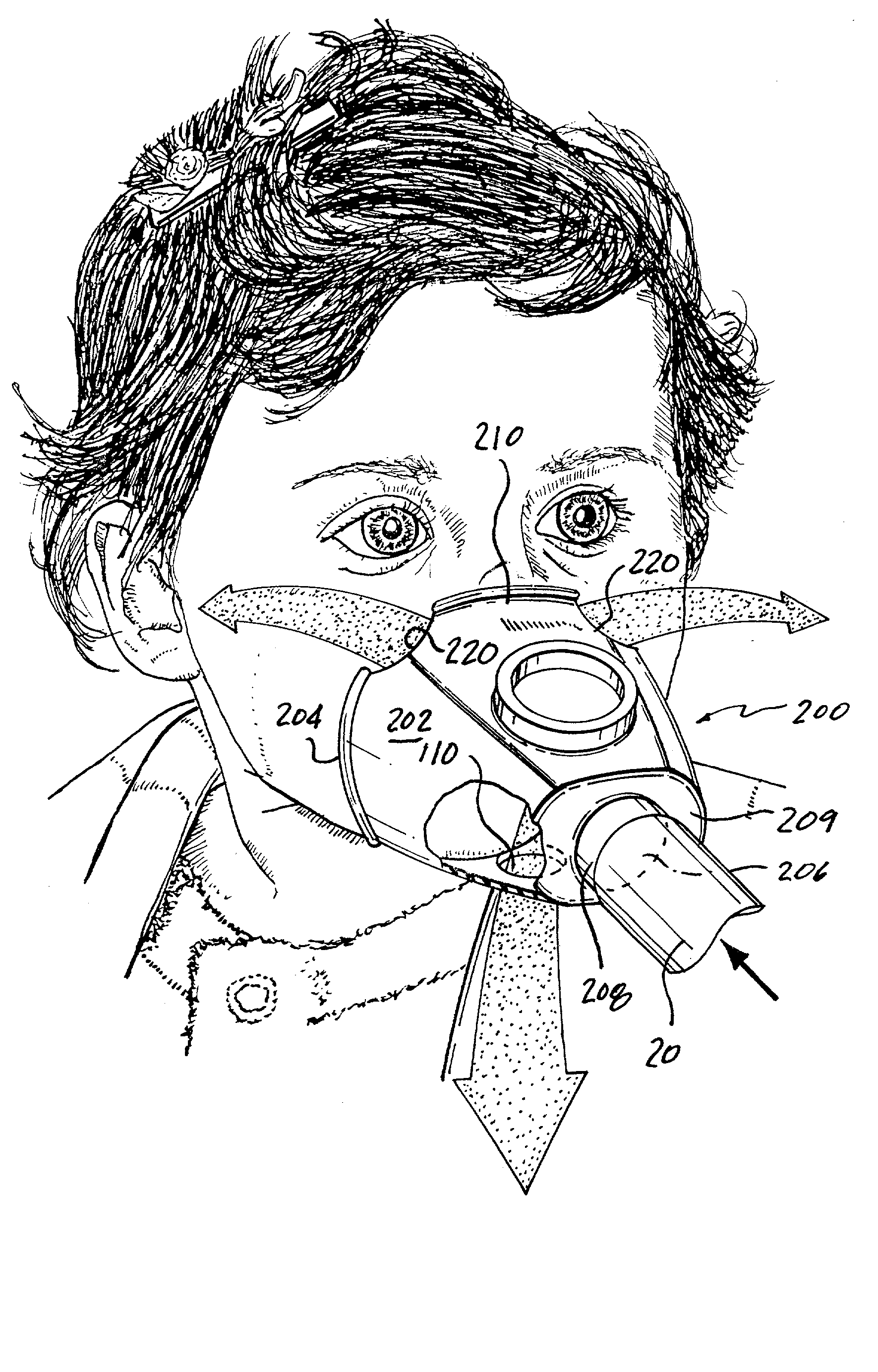
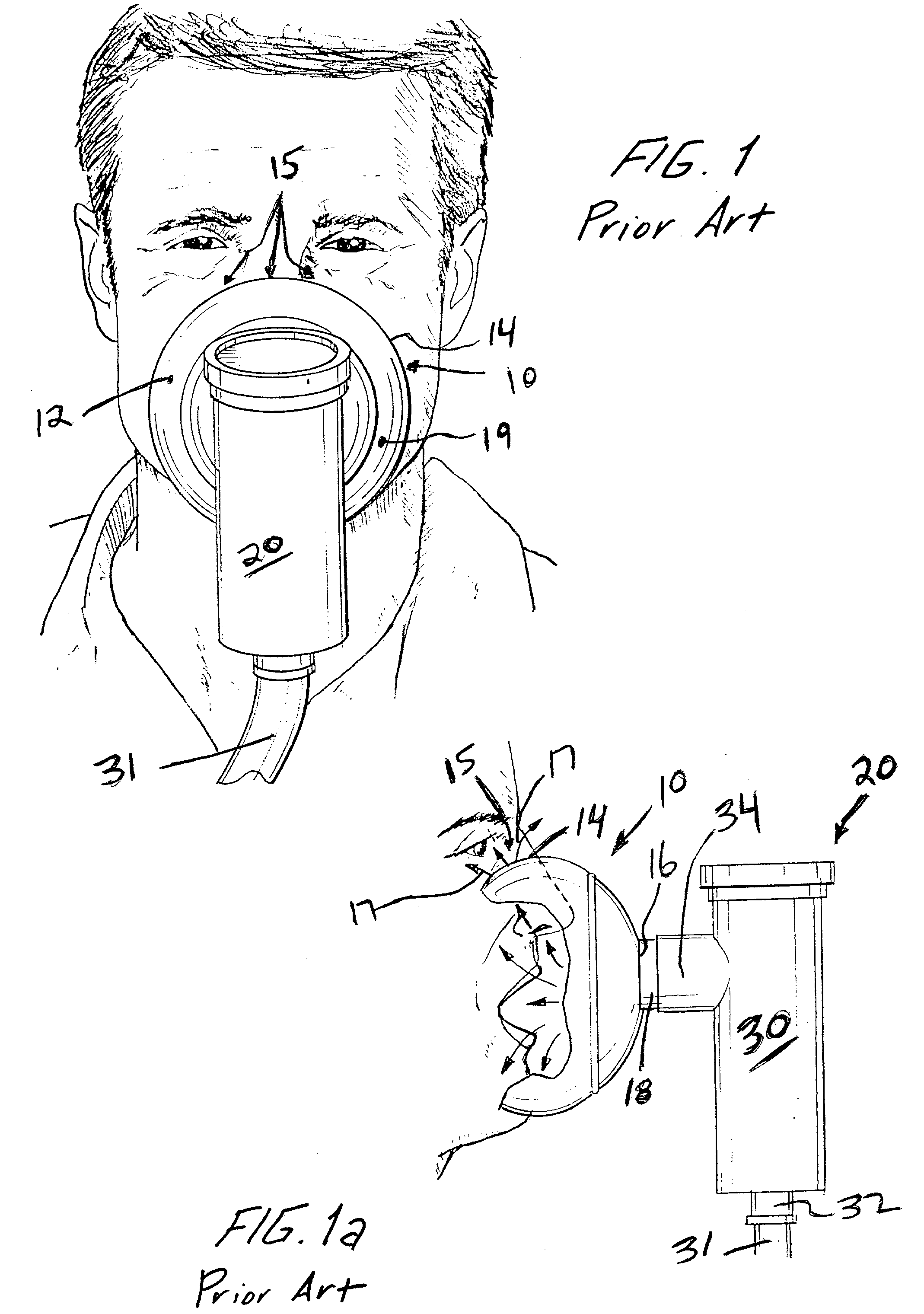
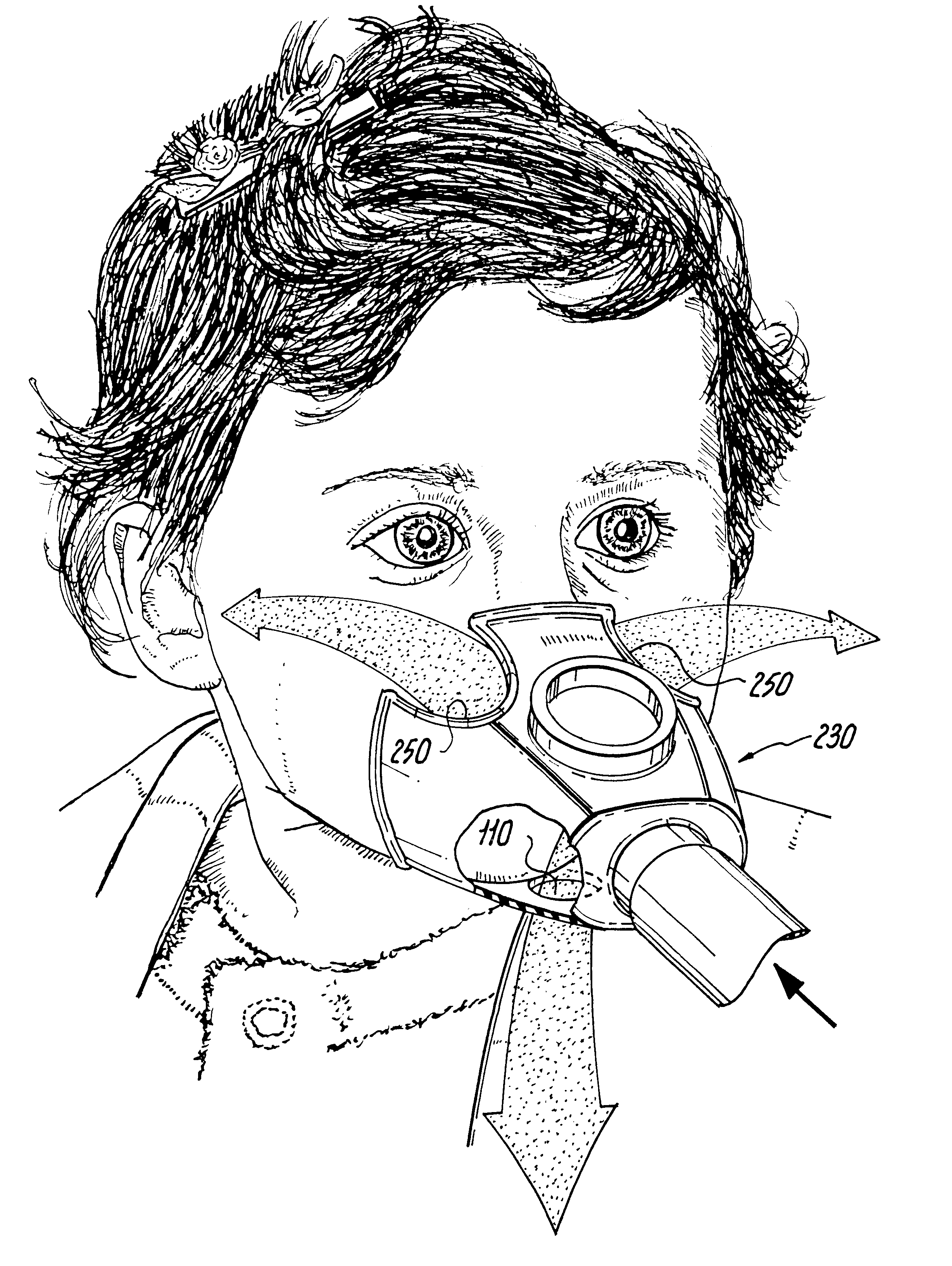
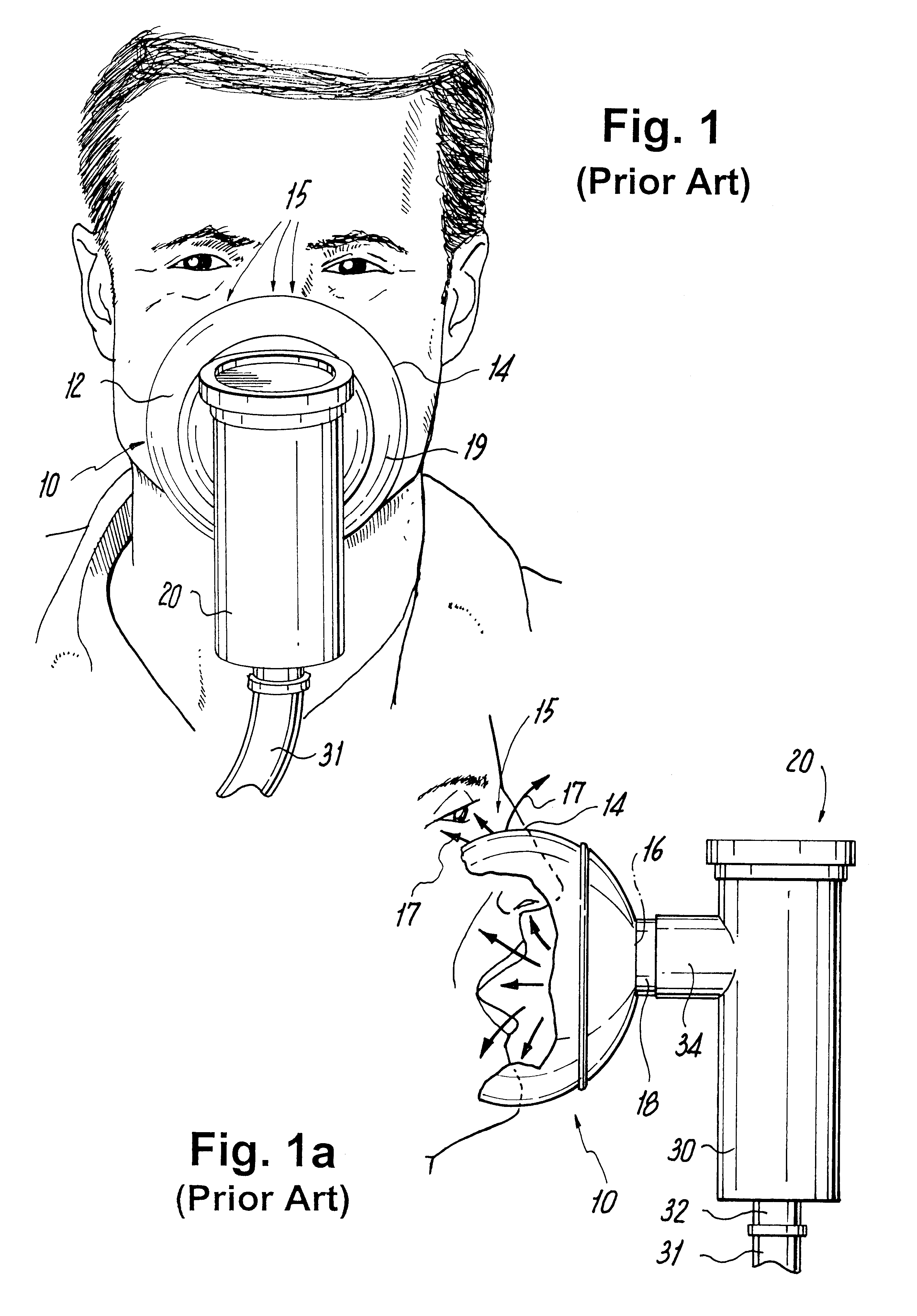
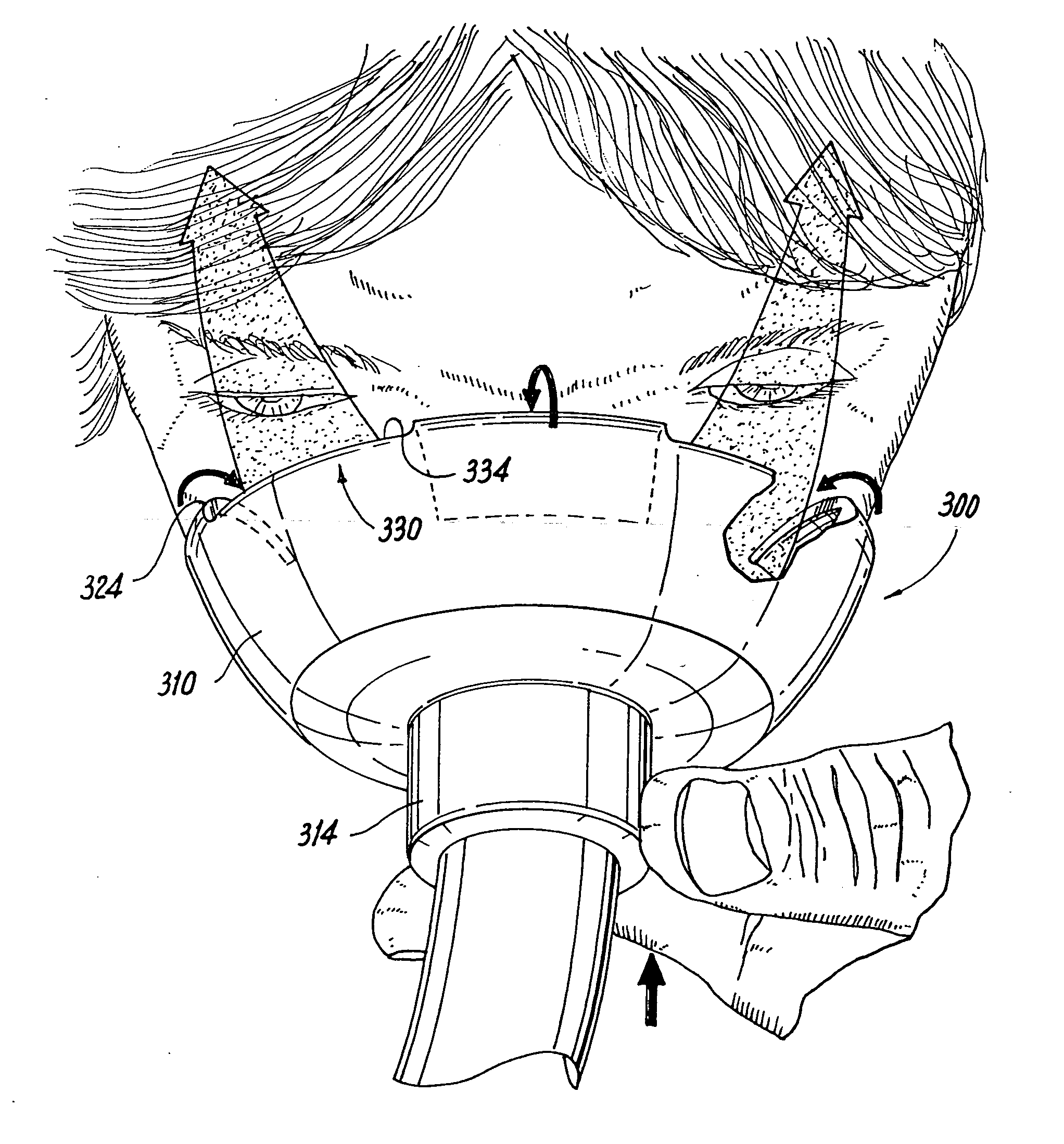
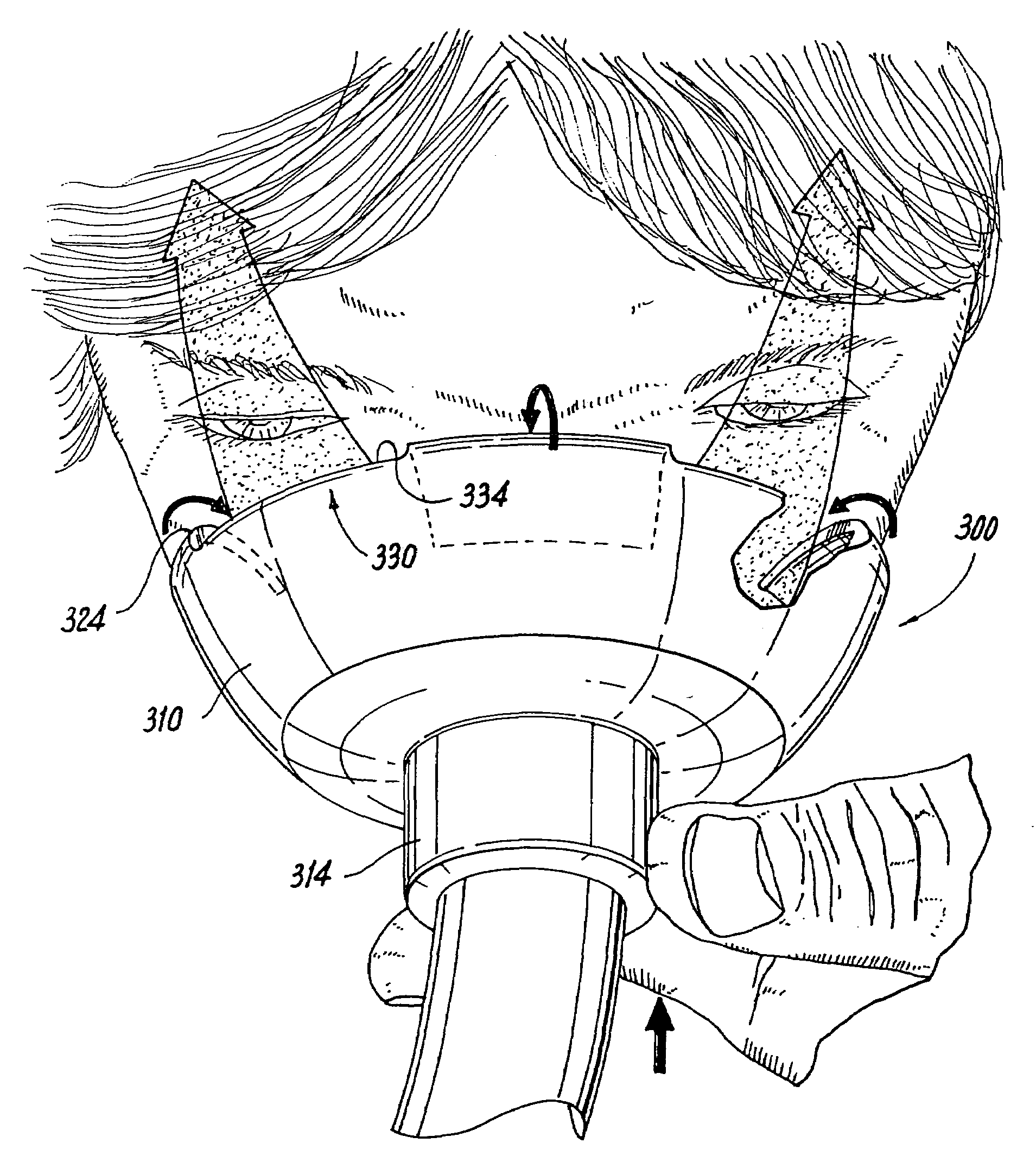
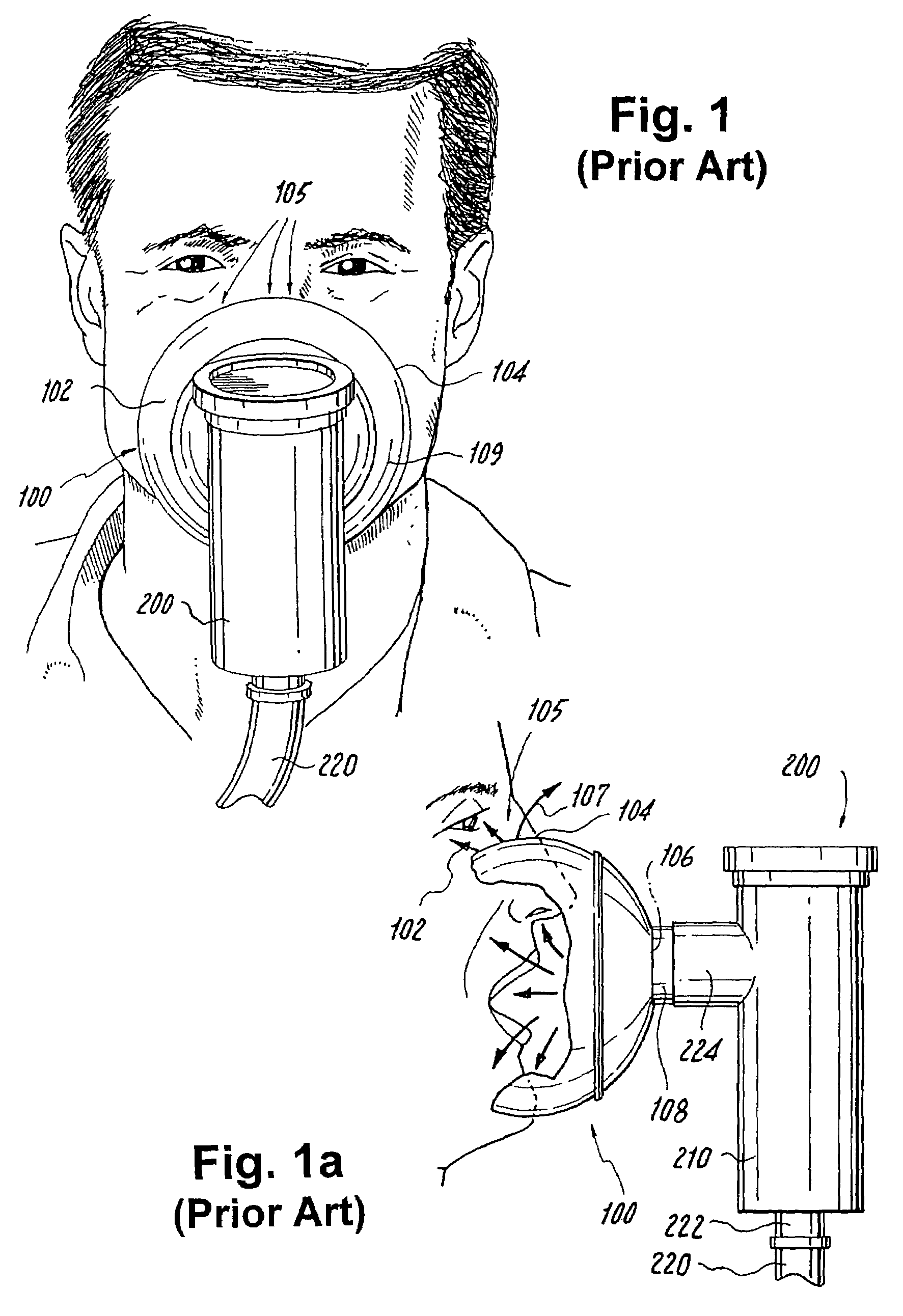
Face masks for use in pressurized drug delivery systems
Face masks for use in pressurized drug delivery applications, such as aerosol drug delivery systems, and a method of reducing aerosol deposition in the region of the eyes are presented. The face masks according to the various embodiments disclosed herein contain features that reduce the inertia of the aerosolized drug in perinasal areas. This results in a reduction in the amount of aerosolized drug that is deposited in the region of the eyes by inertial impaction, while at the same time, the features are constructed to maintain the flow of the aerosolized drug into the face mask so that the aerosolized drug is effectively delivered to the respiratory system of the patient.
Owner:SMALDONE GERALD C
Aerosolized fluoroquinolones and uses thereof
ActiveUS7838532B2Reduce riskHigh levelPowder deliveryHeavy metal active ingredientsAerosol drugsLevofloxacin
Owner:HORIZON ORPHAN LLC
Face masks for use in pressurized drug delivery systems
Face masks for use in pressurized drug delivery applications, such as aerosol drug delivery systems, and a method of reducing aerosol deposition in the region of the eyes are presented. The face masks according to the various embodiments disclosed herein contain features that reduce the inertia of the aerosolized drug in perinasal areas. This results in a reduction in the amount of aerosolized drug that is deposited in the region of the eyes by inertial impaction, while at the same time, the features are constructed to maintain the flow of the aerosolized drug into the face mask so that the aerosolized drug is effectively delivered to the respiratory system of the patient.
Owner:SMALDONE GERALD C
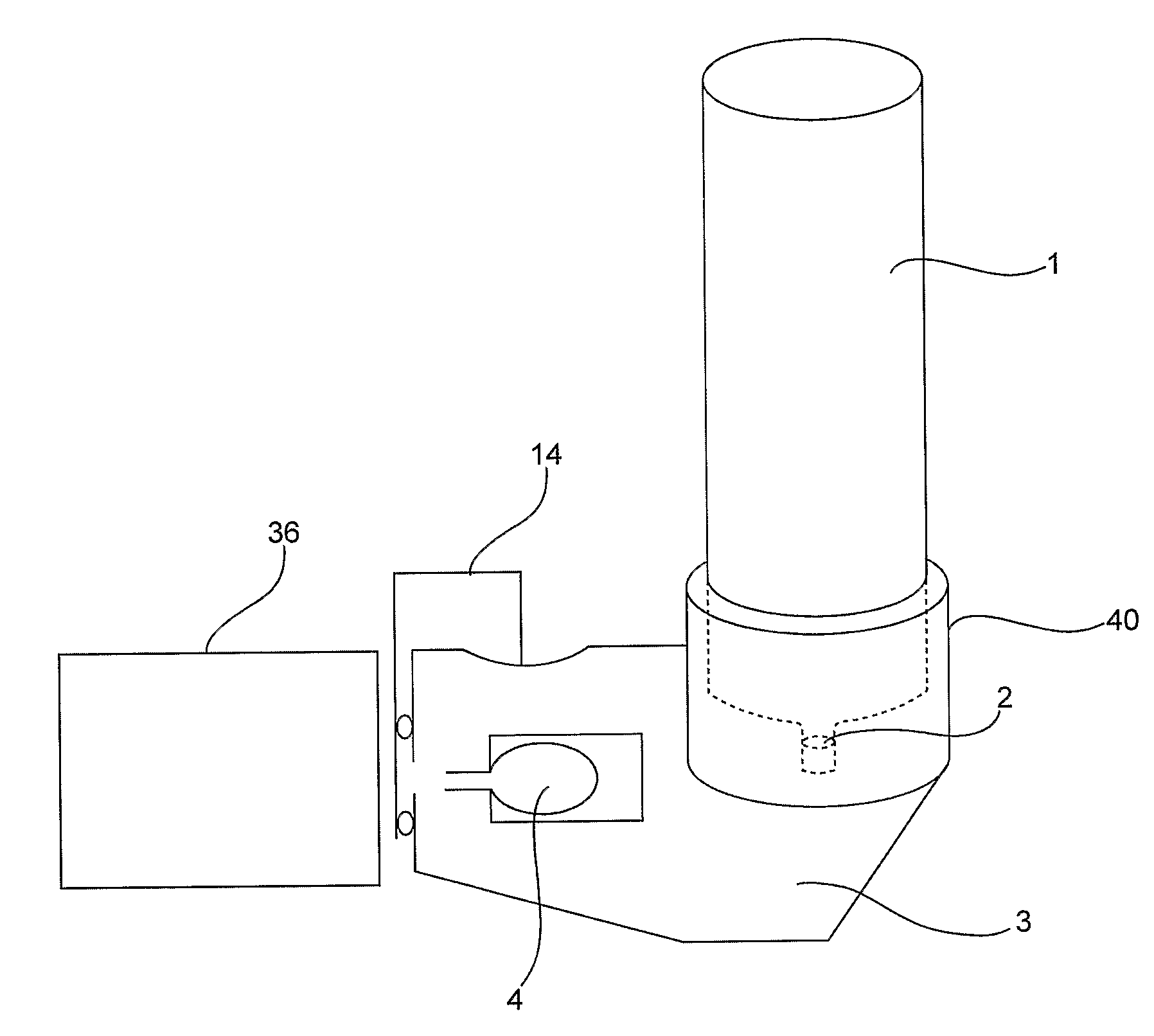
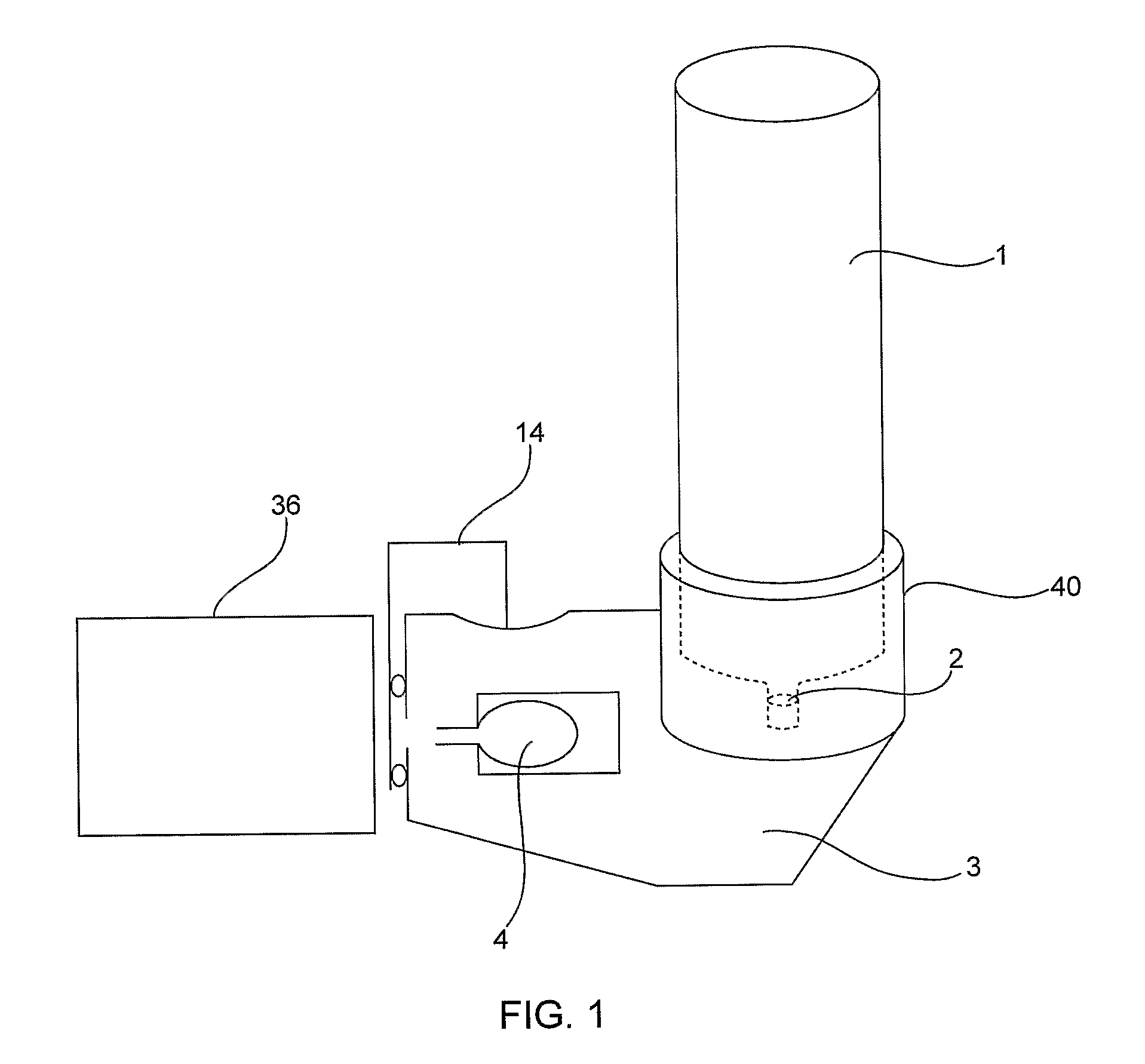
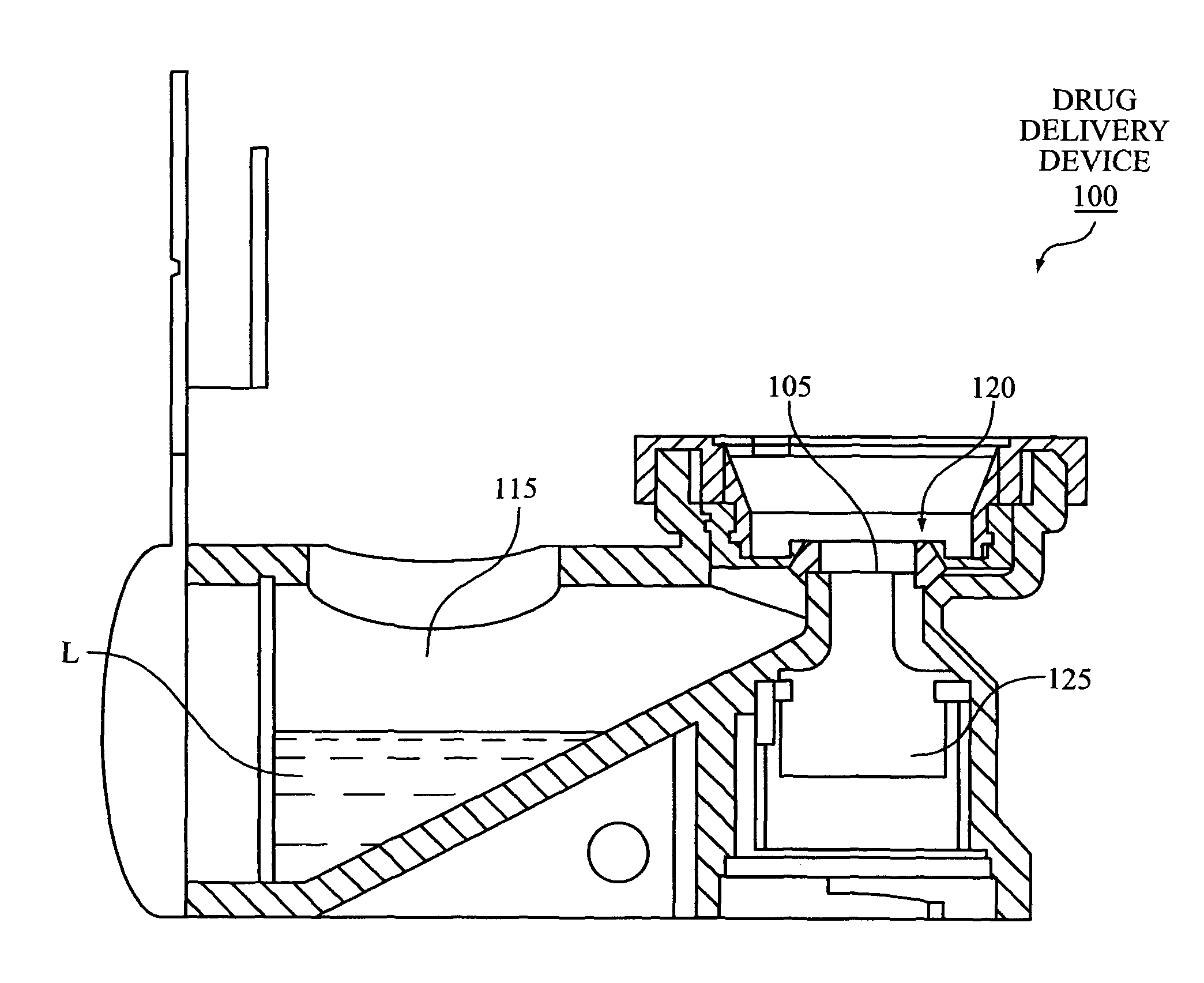
Device and Method for Generating an Aerosol From a Liquid Formulation and Ensuring Its Sterility
A drug delivery device containing a sterile multi dose reservoir. Said sterile reservoir can be used with many types of delivery including injectors or aerosol drug delivery systems. Elevated pressure surrounding the reservoir is used during storage to ensure sterility is maintained. Mechanisms to prevent delivery in the case of potential compromise of sterility are disclosed. A device using the pressure to meter formulation from the reservoir is disclosed.
Owner:ARADIGM
Face mask for use in pressurized drug delivery systems
ActiveUS20050150496A1Reducing aerosol depositionReduce inertiaRespiratory masksBreathing masksNoseAerosol deposition
Face masks for use in pressurized drug delivery applications, such as aerosol drug delivery systems, and a method of reducing aerosol deposition in the region of the eyes are presented. The face masks according to the various embodiments disclosed herein contain features that reduce the inertia of the aerosolized drug in perinasal areas. This results in a reduction in the amount of aerosolized drug that is deposited in the region of the eyes by inertial impaction, while at the same time, the features are constructed to maintain the flow of the aerosolized drug into the face mask so that the aerosolized drug is effectively delivered to the respiratory system of the patient.
Owner:SMALDONE GERALD C
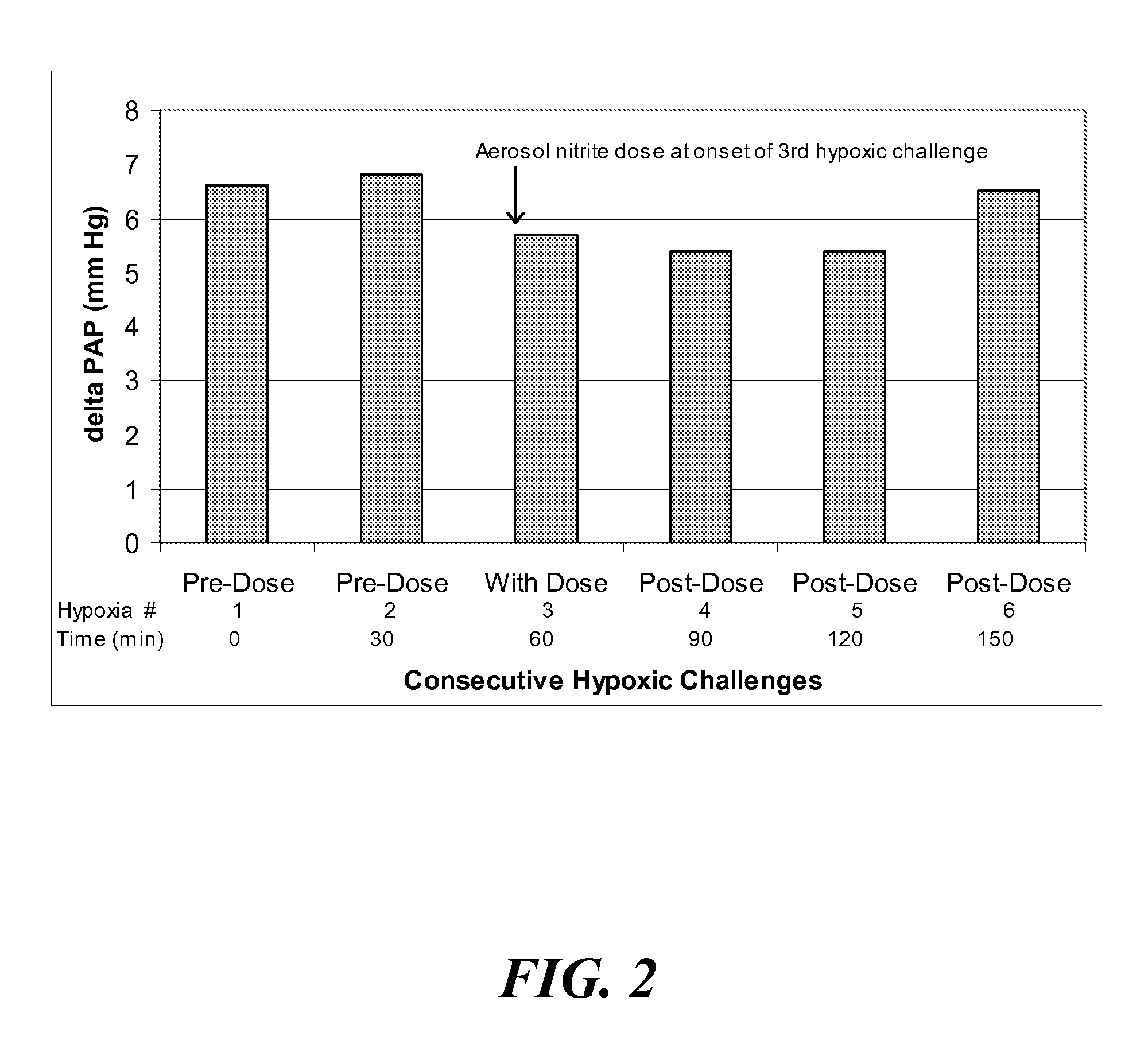
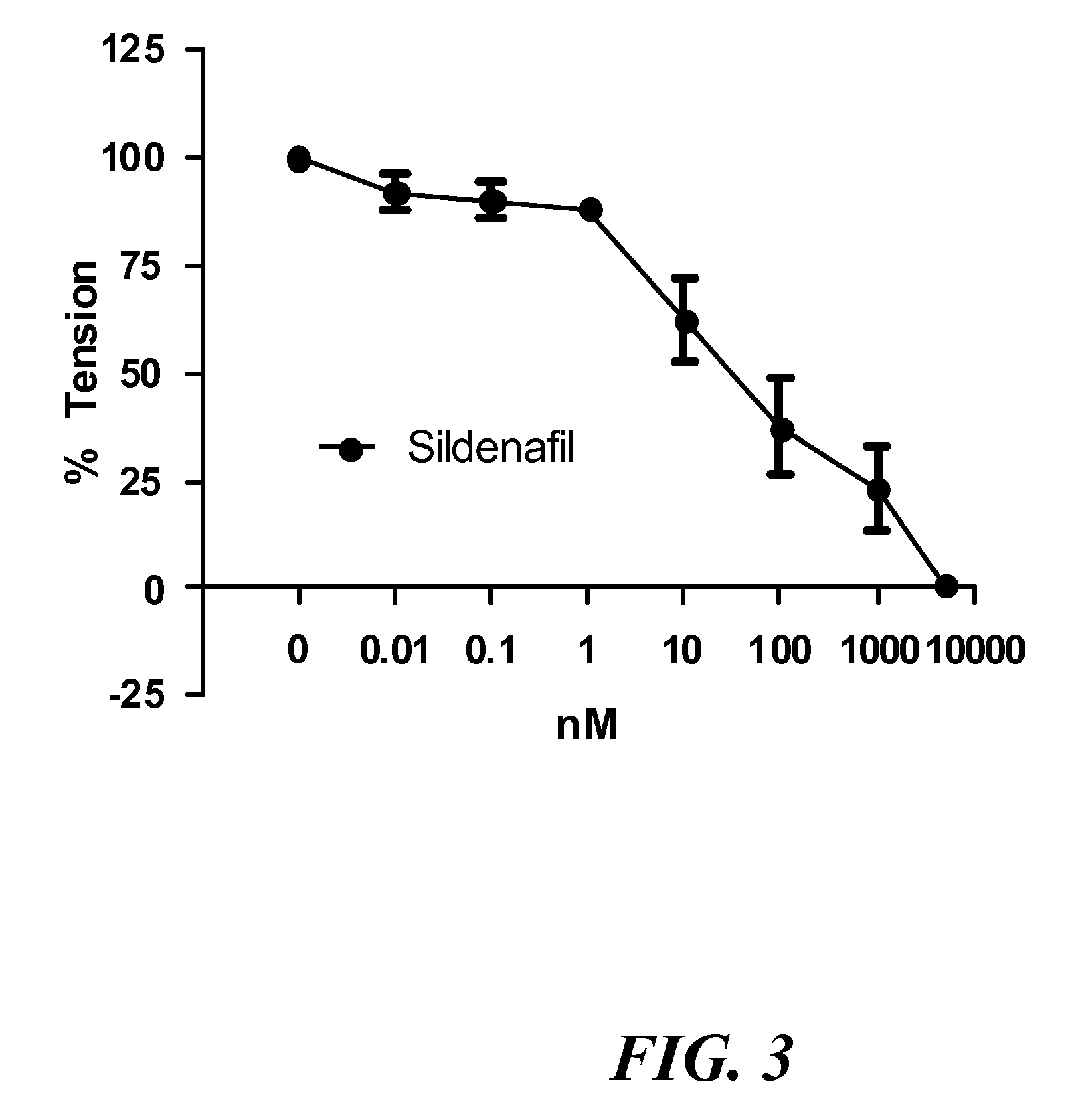
Aerosolized nitrite and nitric oxide-donating compounds and uses thereof
Disclosed herein are formulations of nitrite, nitrite salt, or nitrite- or nitric oxide-producing compounds suitable for aerosolization and use of such formulations for aerosol administration of nitrite, nitrite salt, or nitrite- or nitric oxide-donating compounds for the treatment of pulmonary arterial hypertension, intra-nasal or pulmonary bacterial infections, or to treat or prevent ischemic reperfusion injury of the heart, brain and organs involved in transplantation. In particular, inhaled nitrite, nitrite salt, or nitrite- or nitric oxide-donating compound specifically formulated and delivered to the respiratory tract for the indications is described. Compositions include all formulations, kits, and device combinations described herein. Methods include inhalation procedures and manufacturing processes for production and use of the compositions described.
Owner:AIRES PHARMA
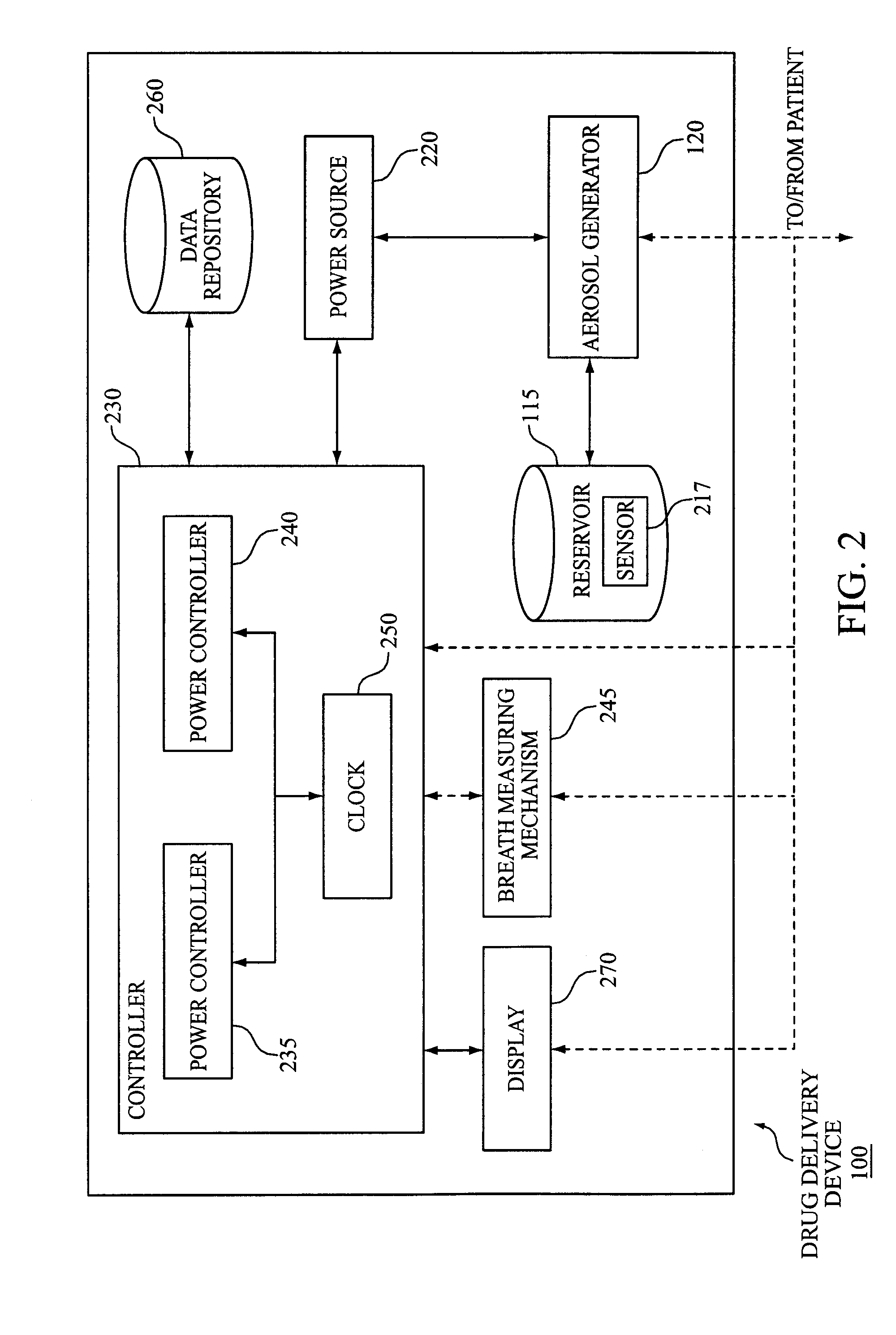
Apparatus and method for maintaining consistency for aerosol drug delivery treatments
An aerosol drug delivery apparatus is provided. The apparatus may include a reservoir constructed to contain a predetermined dose of a liquid drug, an aerosol generator in communication with the reservoir, and a power source arranged to deliver power to the aerosol generator in order to produce an aerosolized form of the dose that can be administered to a patient. The apparatus may maintain consistent aerosol drug delivery treatments, for example, by way of a breath measuring mechanism that monitors the patient's breathing pattern during the administration of the dose and a controller configured to vary the power level at which the power source during the administration of the dose based on the monitored breathing pattern.
Owner:RESPIRONICS RESPIRATORY DRUG DELIVERY UK
Temperature controlling device for aerosol drug delivery
InactiveUS6845216B2Improve drug delivery efficiencyGood repeatabilityRespiratorsMedical devicesTemperature controlMedicine
A portable air temperature controlling device useful for warming air surrounding an aerosolized drug formulation is described. Warming the air of an aerosol makes it possible to reduce the size of aerosol particles produced by an aerosol generation device. Additionally, warming the air forces the size of the aerosol particles to be in the range required for systemic drug delivery independent of ambient conditions. Smaller particles can be more precisely targeted to different areas of the respiratory tract.
Owner:ARADIGM
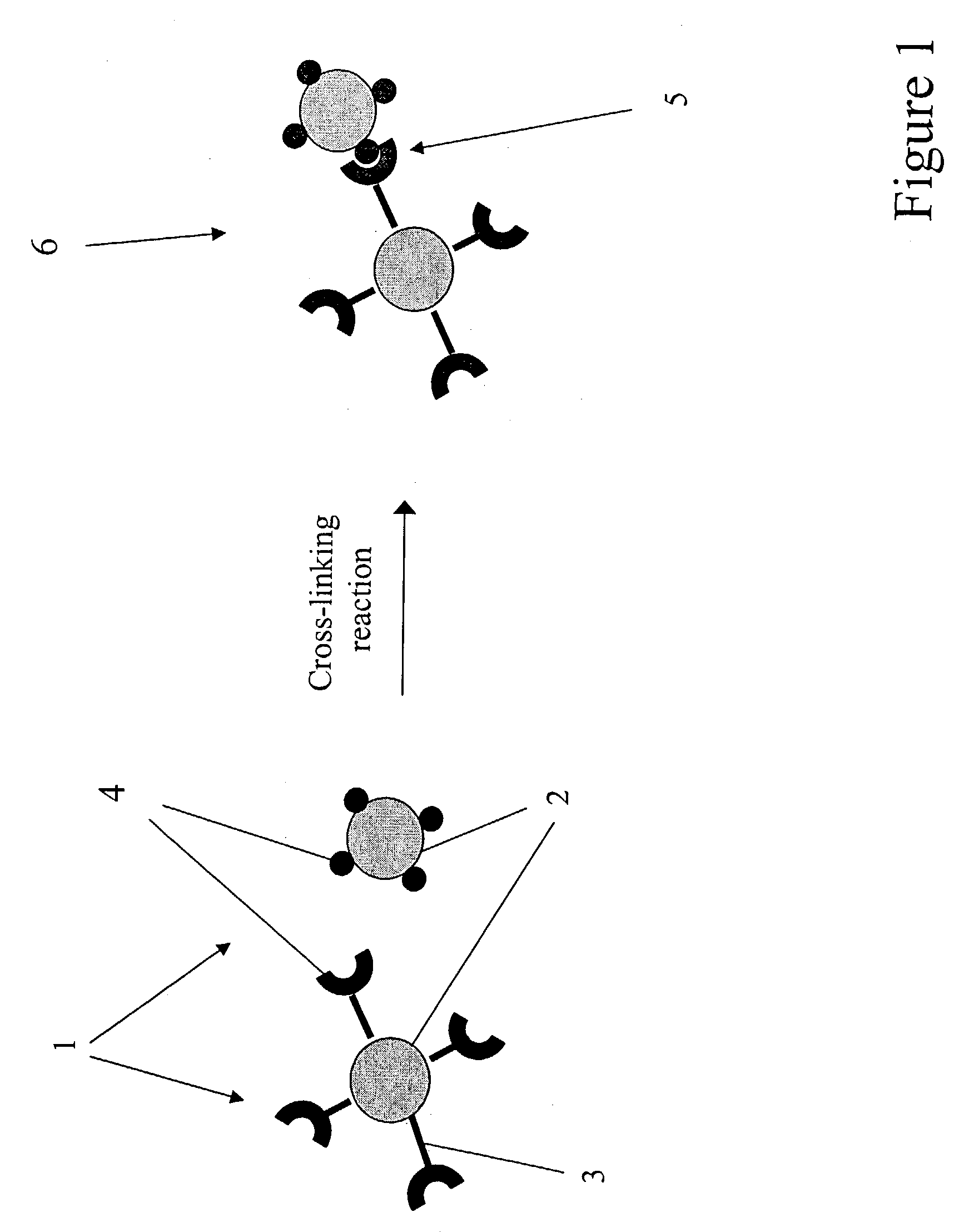
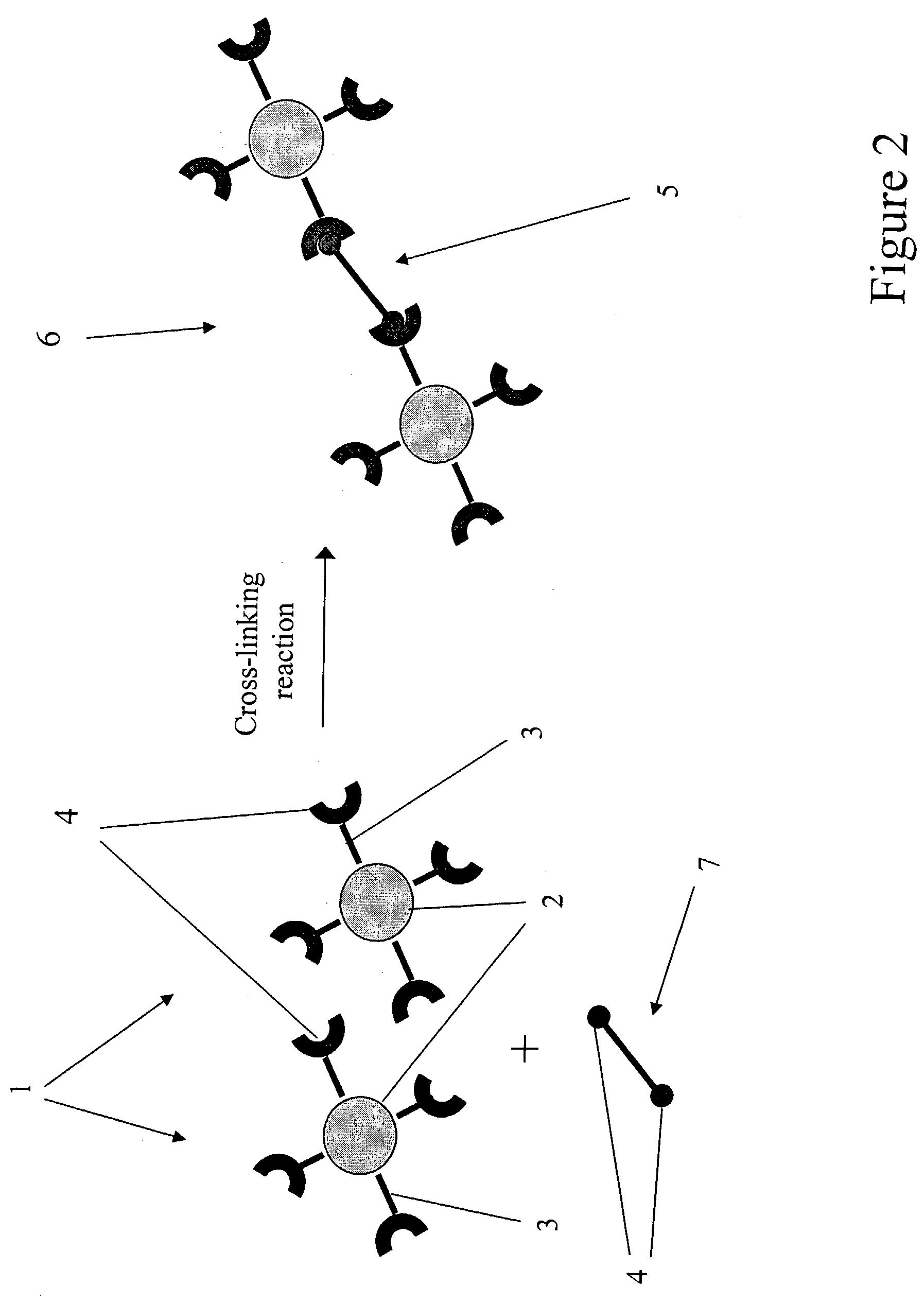
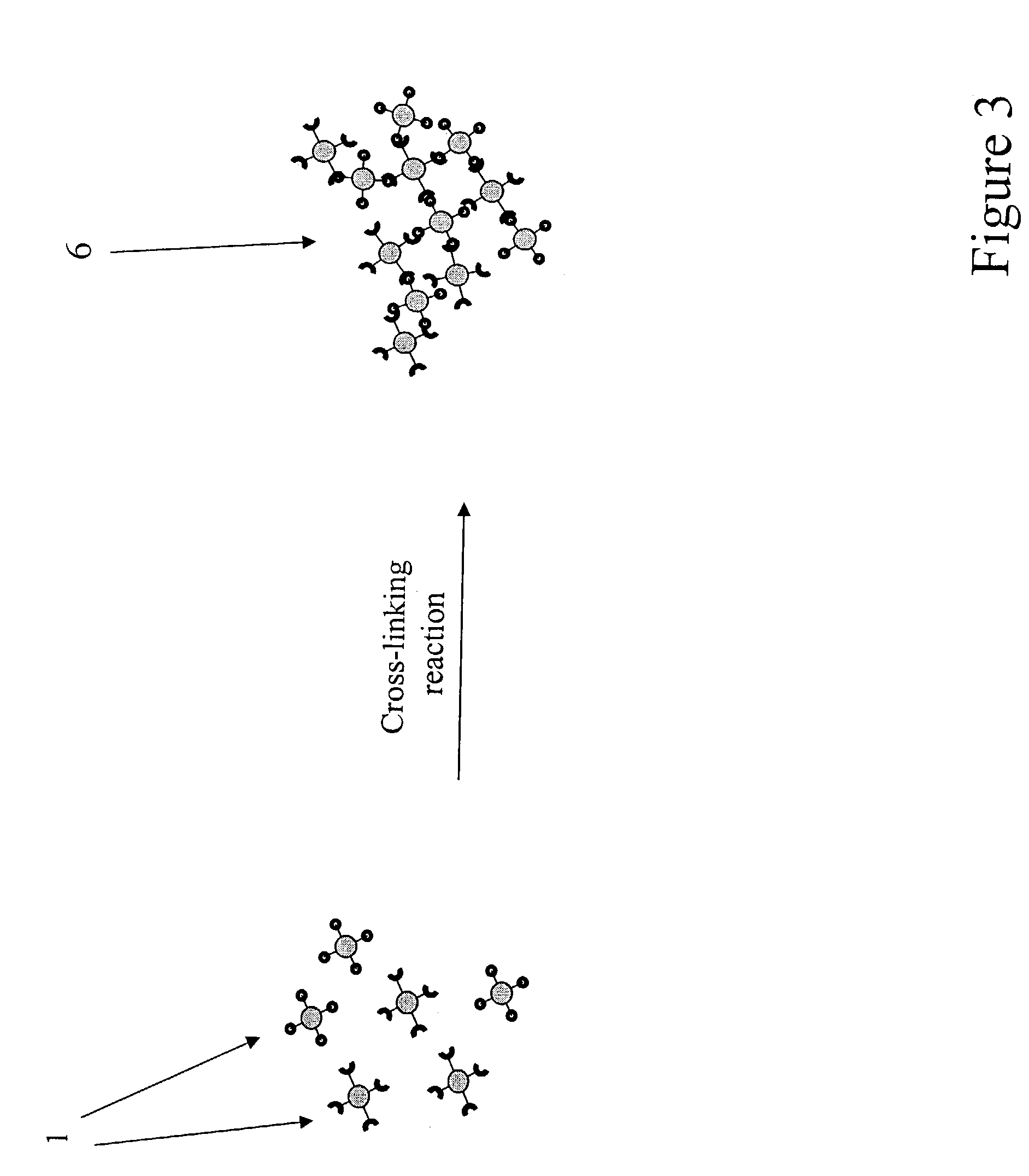
Agglomerated particles for aerosol drug delivery
InactiveUS7138136B2Control releaseControl rateUltrasonic/sonic/infrasonic diagnosticsPowder deliveryDrugAerosol drug delivery
The invention provides a drug delivery vehicle for inhalation by a patient. The drug delivery vehicle comprises biocompatible particles between 0.1 and 1.0 microns in diameter, that are loaded with one or more drugs and which are crosslinked together to form agglomerates. The agglomerates can also provide for delivery of contrast-enhancing agents for clinical imaging. The invention also provides methods of making the inventive agglomerates, pharmaceutical compositions comprising the agglomerates, and methods for delivering drugs or contrast-enhancing agents to a patient by inhalation of pharmaceutical compositions containing the agglomerates.
Owner:CLEVELAND STATE UNIVERSITY
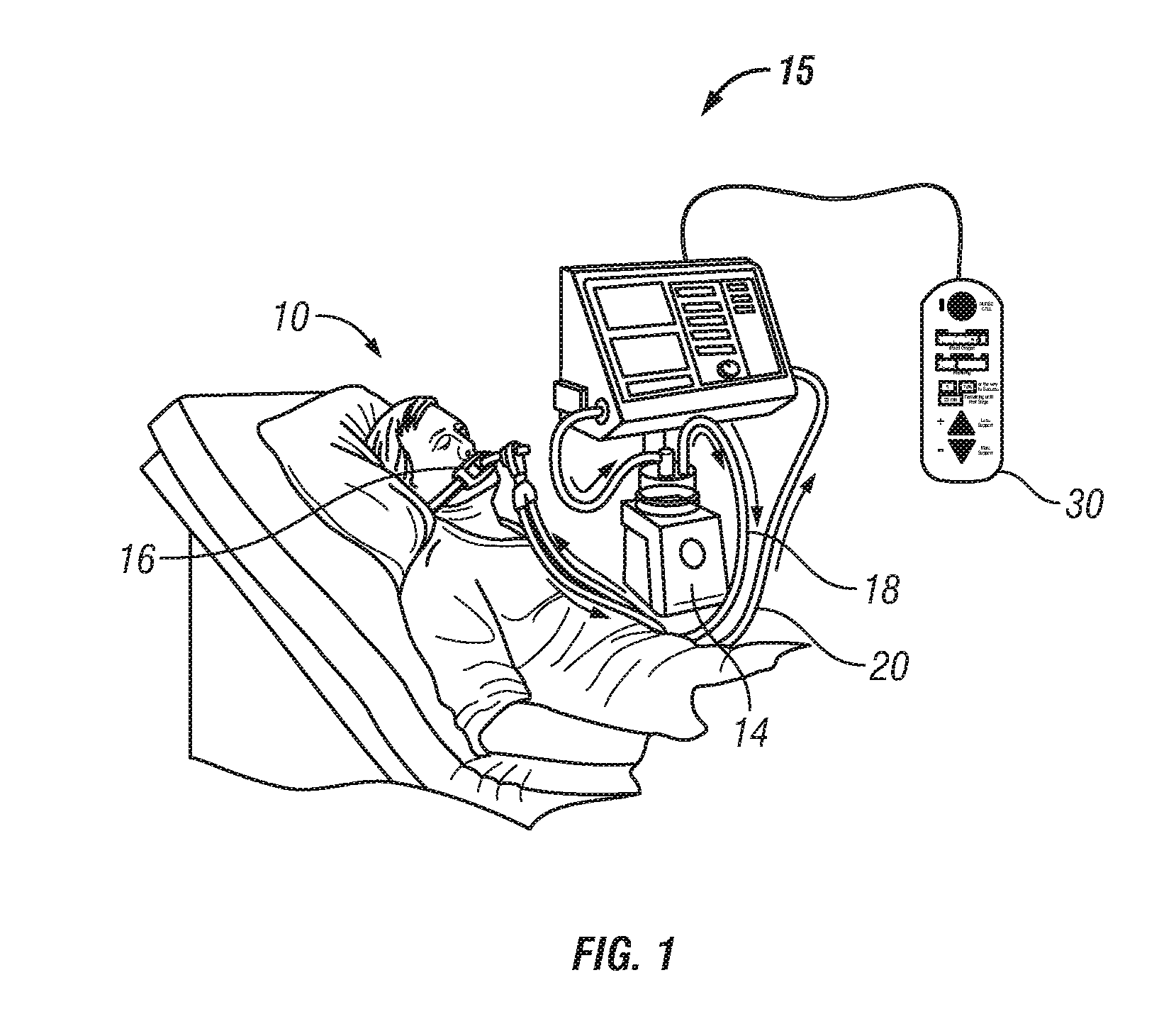
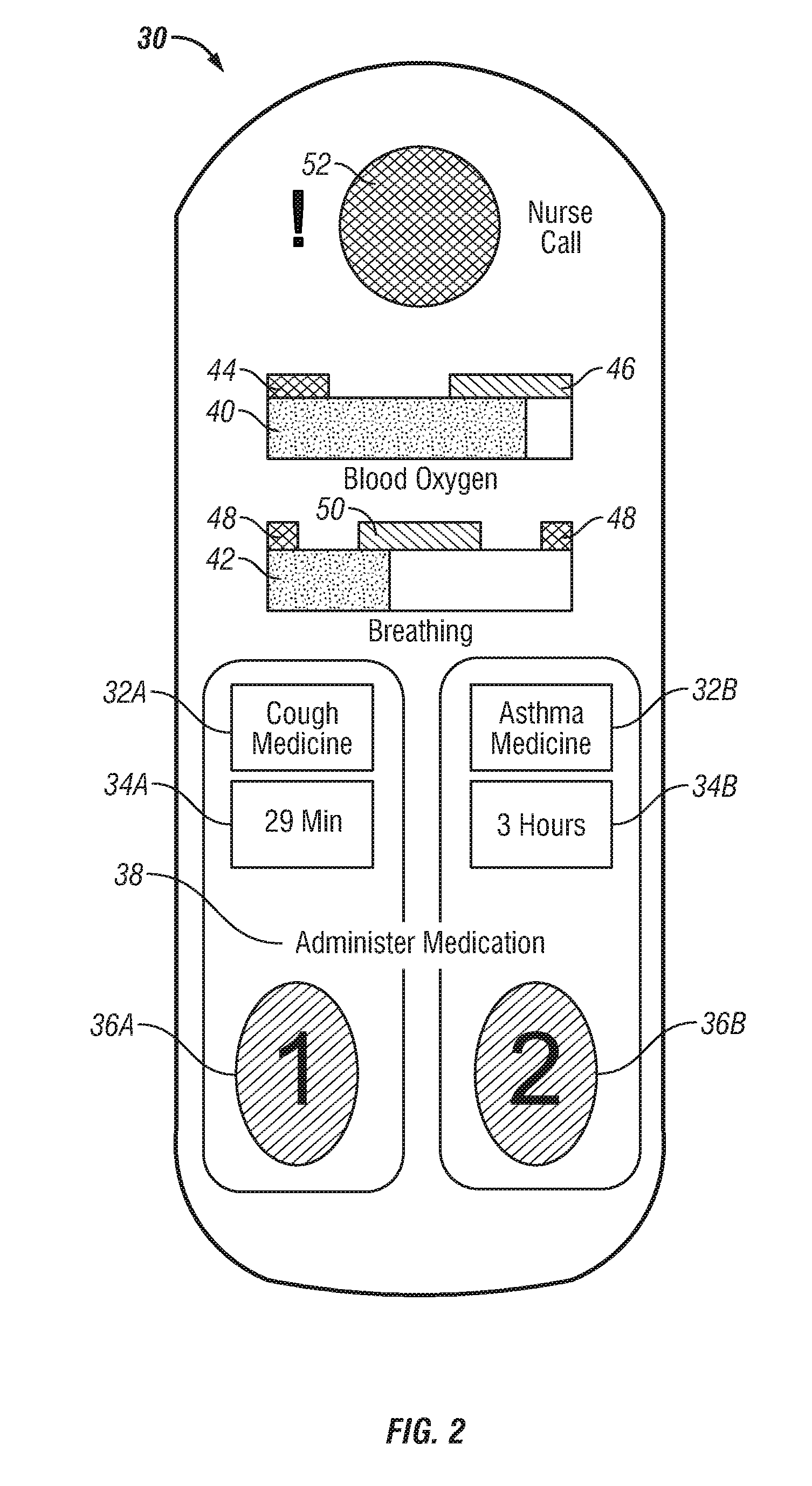
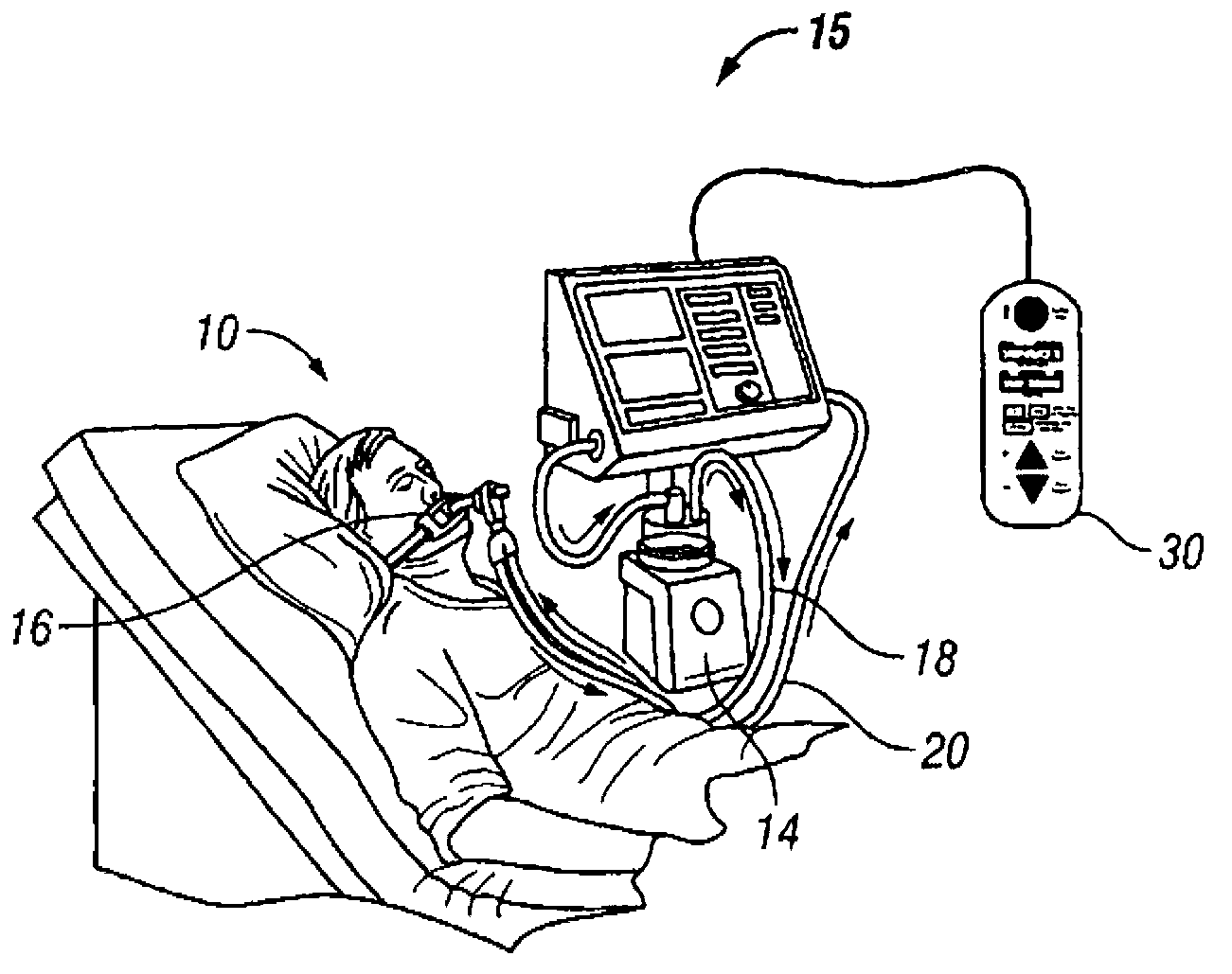
Patient-controlled aerosol administration
A system and method of controlling the administration of a medical substance is disclosed. An aerosol generator is configured to aerosolize a medical substance and administer the aerosolized medical substance to a patient using a ventilator. The patient is provided with a patient control interface through which the patient initiates the administration of a dose of the aerosolized medical substance. A processor is configured to control the ventilator and the aerosol generator in response to the patient control interface such that the patient controls the administration of the aerosolized medical substance in accordance with limits on the administration of the medical substance.
Owner:VYAIRE MEDICAL CAPITAL LLC
Treating cystic fibrosis with antibiotics via an aerosol drug
A method of treating respiratory disorders by delivering antibiotic to the lung alveoli using an aerosol drug delivery system.
Owner:WYETH LLC
Aerosolized fluoroquinolones and uses thereof
ActiveUS20100158957A1Reduce riskHigh levelAntibacterial agentsPowder deliveryAerosol drugsLevofloxacin
Disclosed herein are formulations of fluoroquinolones suitable for aerosolization and use of such formulations for aerosol administration of fluoroquinolone antimicrobials for the treatment of pulmonary bacterial infections. In particular, inhaled levofloxacin specifically formulated and delivered for bacterial infections of the lungs is described. Methods include inhalation protocols and manufacturing procedures for production and use of the compositions described.
Owner:HORIZON ORPHAN LLC
Aerosol for treating faucitis and preparation method thereof
InactiveCN101987165AEasy to administerQuick effectAnthropod material medical ingredientsHydroxy compound active ingredientsCurative effectChronic pharyngolaryngitis
The invention provides aerosol for treating faucitis and a preparation method thereof. The aerosol is convenient to administrate, and has good curative effect of treating the faucitis, in particular treating hoarseness, aphonia and the like.
Owner:JIANGXI JEMINCARE GRP CO LTD
Face mask for use in pressurized drug delivery systems
InactiveUS7082947B2Reducing aerosol depositionReduce inertiaRespiratory masksBreathing masksAerosol depositionAerosol drug delivery
Owner:SMALDONE GERALD C
Aerosolized fluoroquinolones and uses thereof
ActiveUS20100040560A1Overcome resistancePrevent further resistanceAntibacterial agentsBiocideInhalationLevofloxacin
Disclosed herein are formulations of fluoroquinolones suitable for aerosolization and use of such formulations for aerosol administration of fluoroquinolone antimicrobials for the treatment of pulmonary bacterial infections. In particular, inhaled levofloxacin specifically formulated and delivered for bacterial infections of the lungs is described. Methods include inhalation protocols and manufacturing procedures for production and use of the compositions described.
Owner:HORIZON ORPHAN LLC
Aerosol feeder pushing button with counter
PendingCN110559528AKnow at any timeReadings show stable and reliableMedical devicesMedical atomisersEngineeringAerosol drug delivery
The invention discloses an aerosol feeder pushing button with a counter. The aerosol feeder pushing button comprises a pushing button, a cap body, a counter and a counter housing. The counter is arranged on an outer side wall of the pushing button. The counter comprises a counter base, a gear, a single-digit dial and a tens-digit dial. The counter base is inserted in the outer side wall of the pushing button. A fixed shaft is arranged on the outer side wall of the pushing button. The single-digit dial is sleeved on the outer side of the fixed shaft. The tens-digit dial is sleeved on the top ofthe single-digit dial . The counter base comprises a cantilever beam, an actuating rod below the cantilever beam and a connecting arm for connecting the cantilever beam and the actuating rod. The endof the actuating rod has an outward protruding end. The one end of the cantilever beam is connected to the counter base and the other end of the cantilever beam is impending. An actuating pawl is arranged at the impending end of the cantilever beam. By the arrangement, the aerosol feeder pushing button can solve the problem that an existing user of aerosol can not know usage count and remaining usage count of the aerosol.
Owner:万通(苏州)定量阀系统有限公司
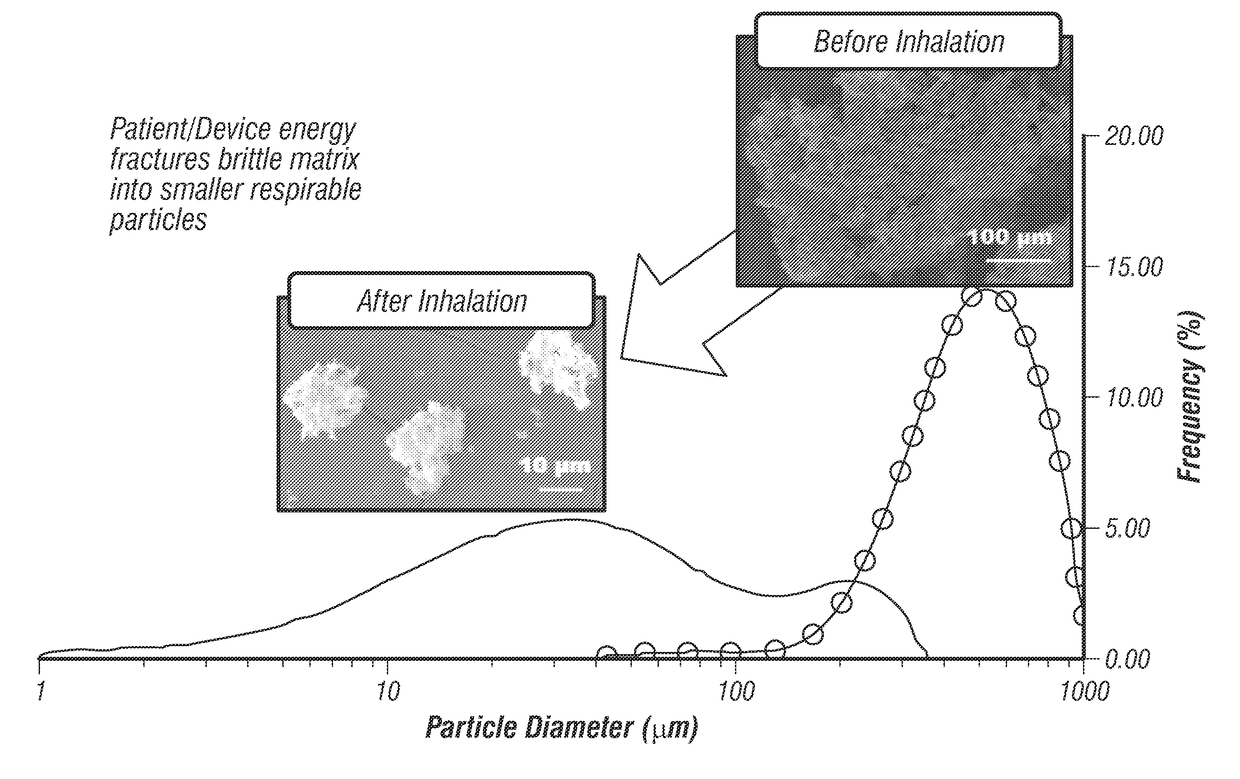
Multidrug brittle matrix compositions
PendingUS20180147161A1Sufficient effectPowder deliveryOrganic active ingredientsDiseaseRespiratory disease
Dual and triple therapy combinations of drugs formulated as brittle matrix particles with a high surface area are provided herein. These particle formulations may be used in inhalation or aerosol administration techniques to provide the drug combinations to the lungs. In some aspects, these compositions may be used to treat a respiratory disease or disorder such as asthma or COPD.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Patient-controlled aerosol administration
Owner:CAREFUSION 303 INC
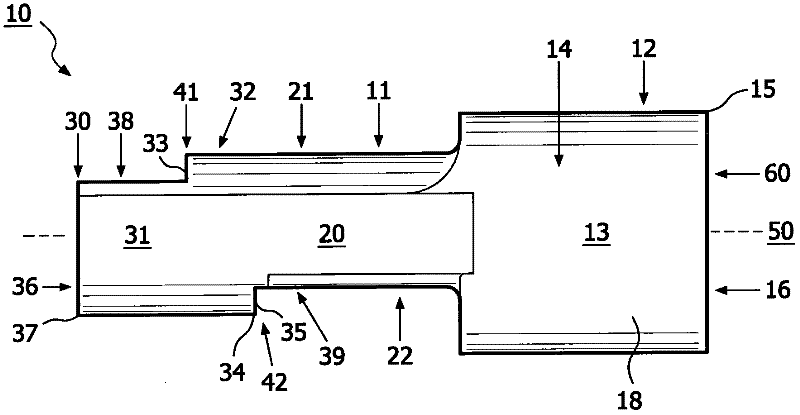
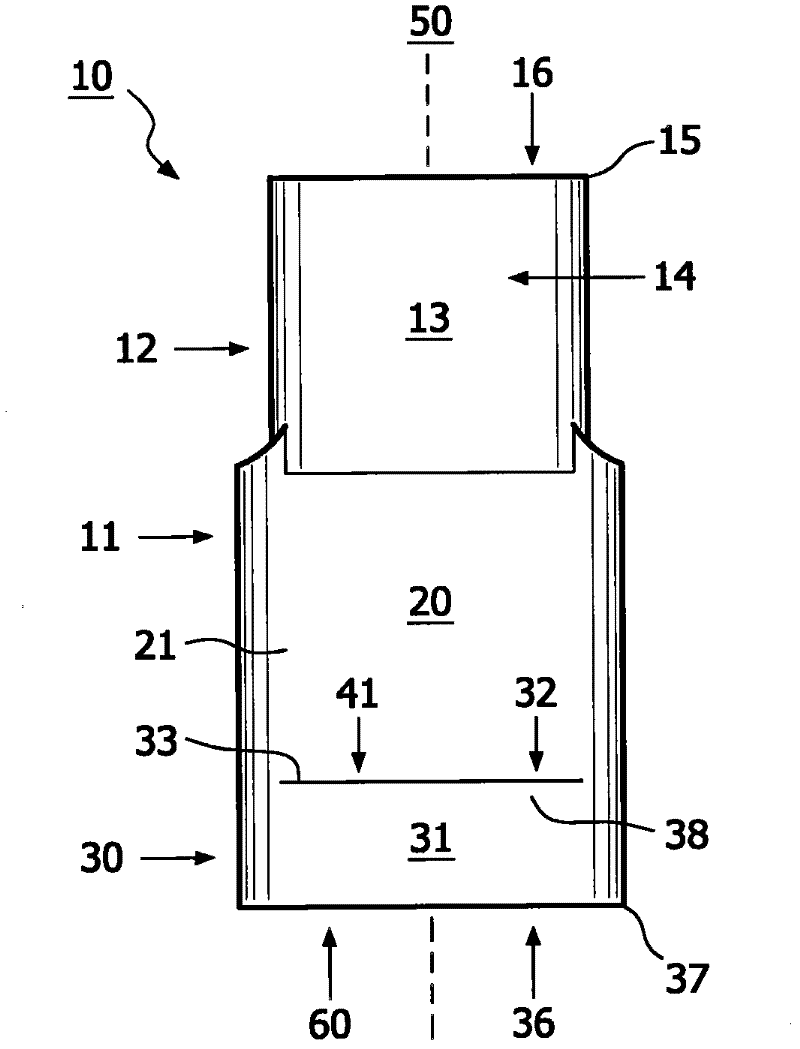
Method for aerosol drug delivery and device including stepped mouthpiece
The present invention relates to an apparatus (10) to aid in administering inhaled pharmaceutical aerosol to a patient. The apparatus (10) is used in conjunction with an aerosol delivery device. The apparatus comprises steps (32, 34) on the top and bottom of the apparatus (10), which when used aid the patient causes mandibular advancement, and opening of the mouth, causing opening of patient's airway, resulting in improved aerosol lung deposition. The invention also relates to a method of using such apparatus in a combination with an aerosol delivery device or a system, and to the mouthpiece of said apparatus.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Device for oral administration of an aerosol for the rhinopharynx, the nasal cavities or the paranasal sinuses
A device for administration of an aerosol includes a generator of particles of size between 10 nm and 200 um, a mouthpiece or mouth mask for oral administration of the aerosol during the nasal exhalation phase or during the respiratory pause phase preceding nasal exhalation, and a source of gas or pressure for conveying the particles. The mouthpiece is airtight, extends beyond the teeth of the patient by a maximum length of 4 cm, and administers the aerosol for the nasal cavities, the rhinopharynx or the paranasal sinuses during aerosol administration phases, such that the is successively applied to the mouth, the rhinopharynx and then the nasal fossae and the sinuses, and then the aerosol escapes via one or both of the patient's nostrils. The device does not allow exhalation via the mouth during aerosol administration phases, and the aerosol particles not being directed to the lungs.
Owner:LA DIFFUSION TECHN FR +1
Temperature controlling device for aerosol drug delivery
InactiveUS20070062526A1Improve efficiencyGood repeatabilityRespiratorsMedical devicesTemperature controlMedicine
Owner:ARADIGM
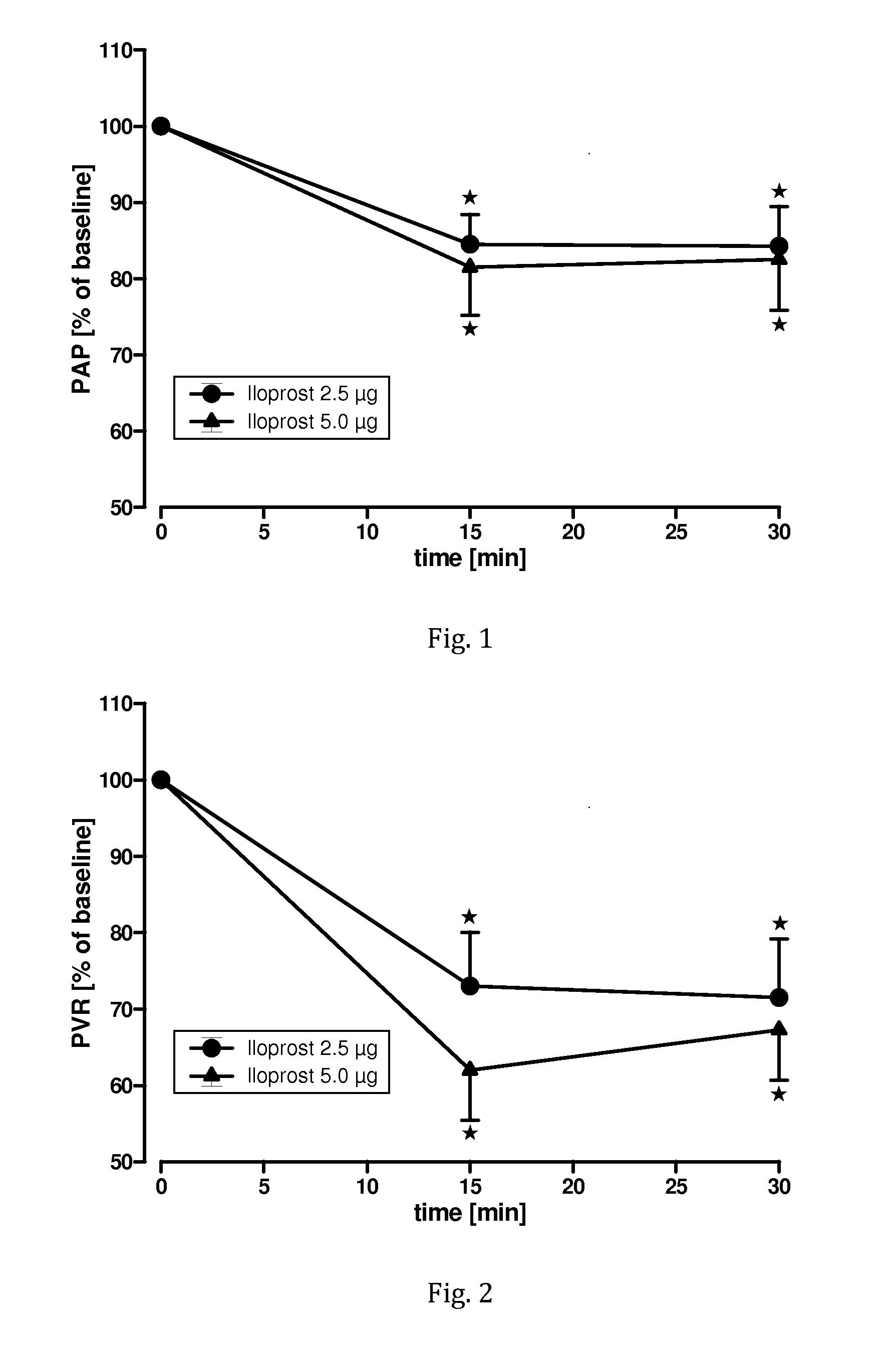
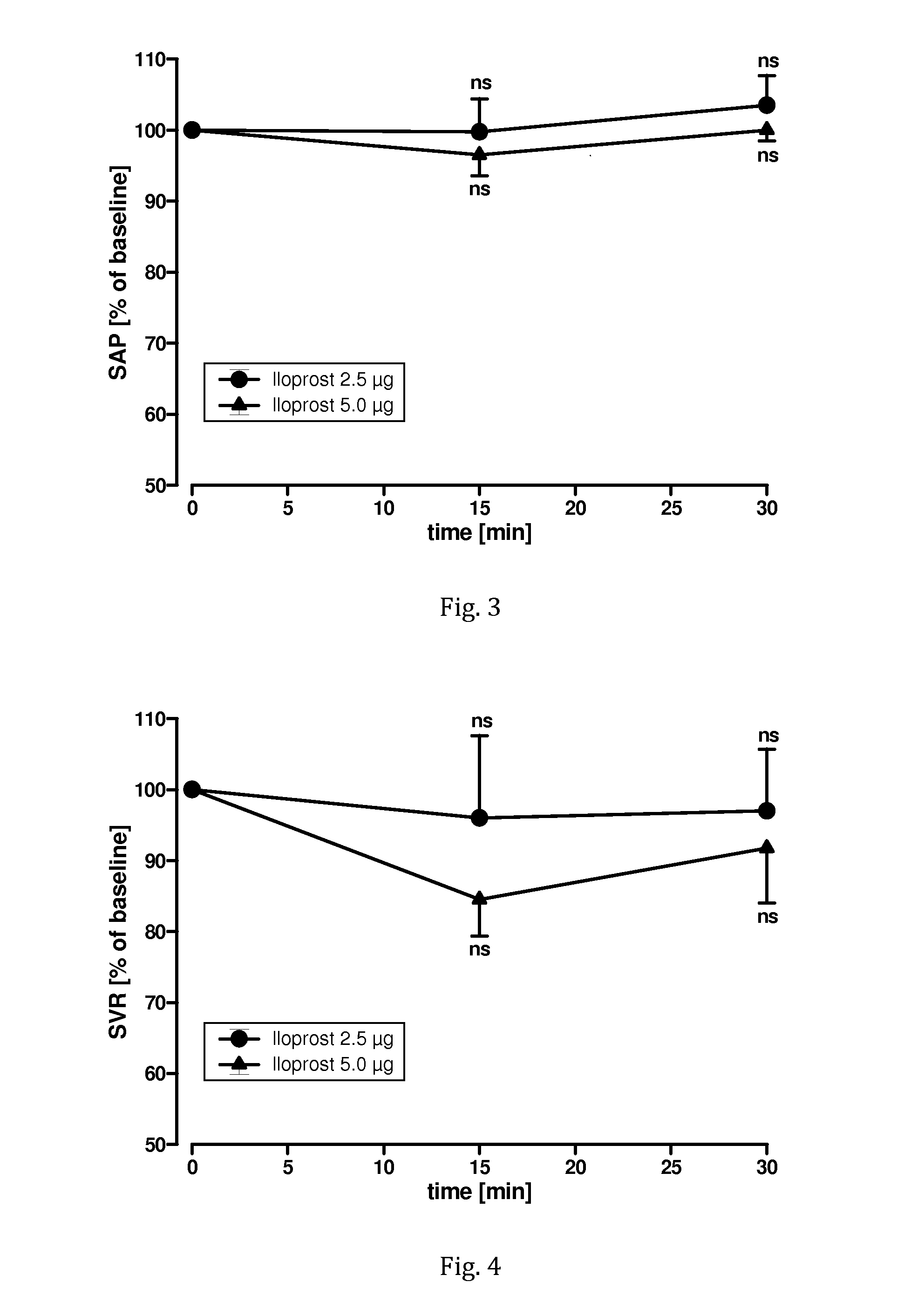
Administration of iloprost as aerosol bolus
ActiveUS20170014424A1Increase productivityImprove efficiencyRespiratorsOrganic active ingredientsDiseaseInhalation
Owner:VECTURA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com