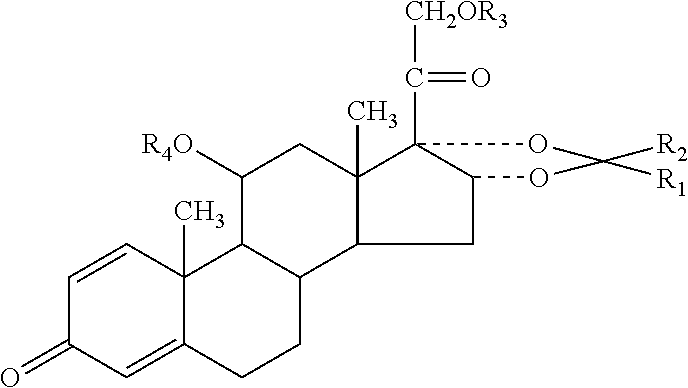
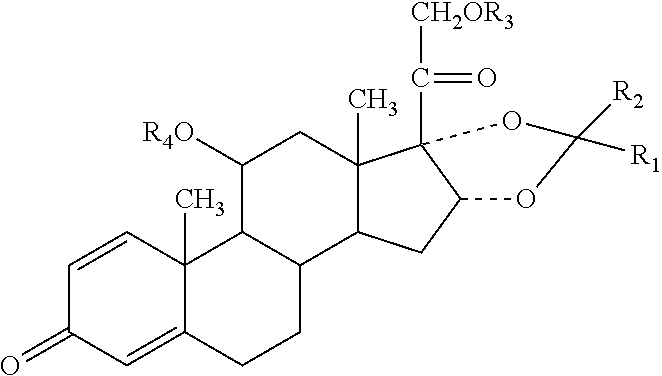
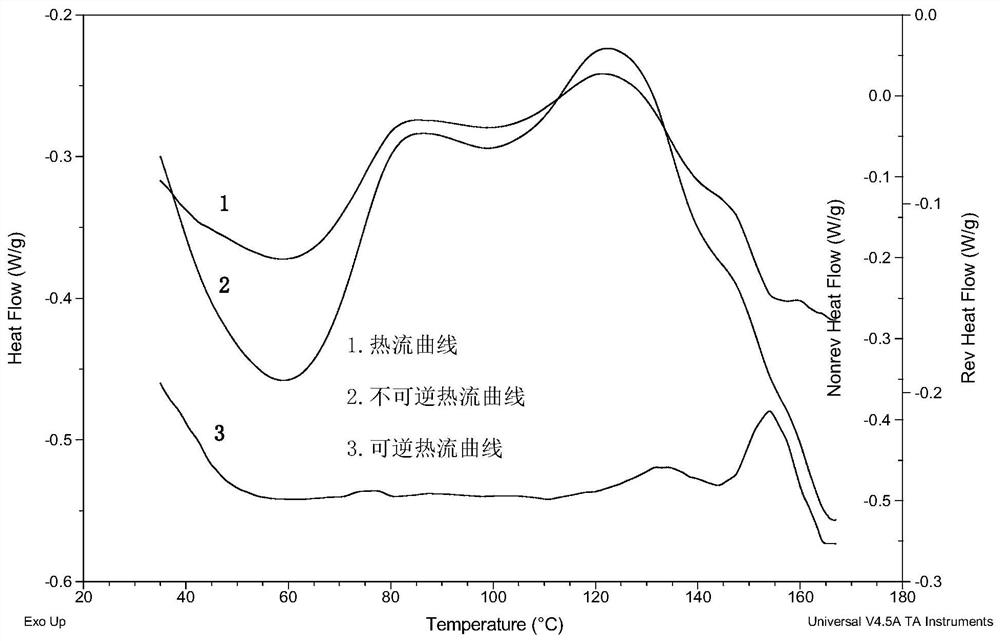
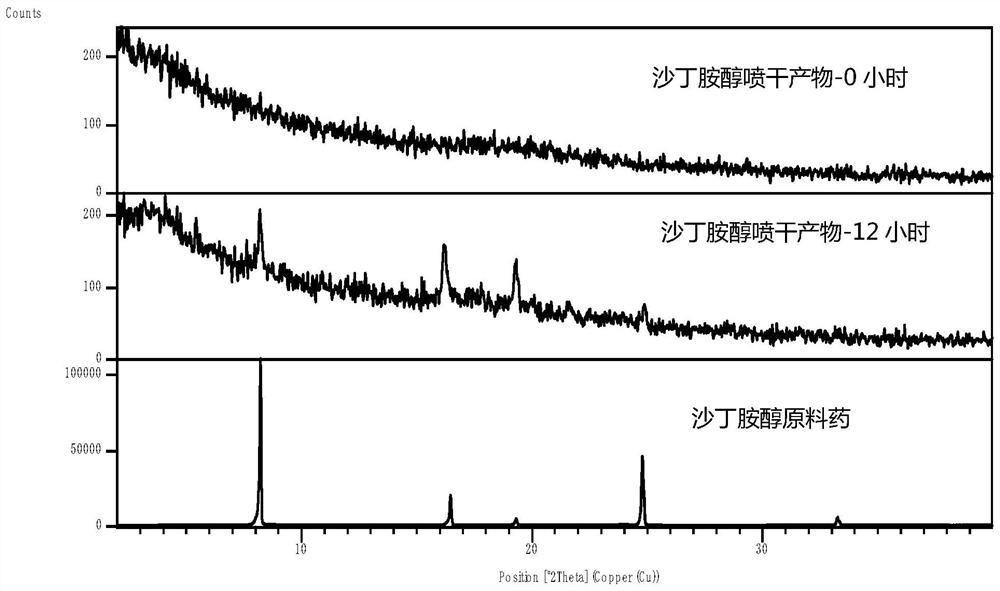
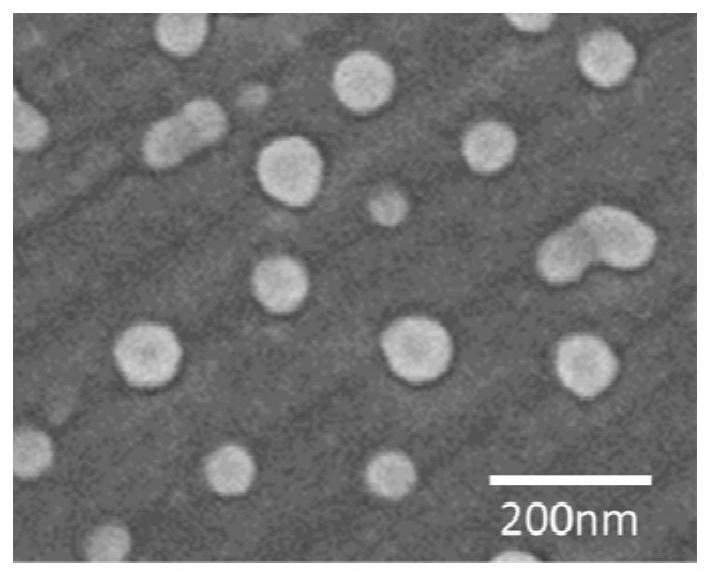
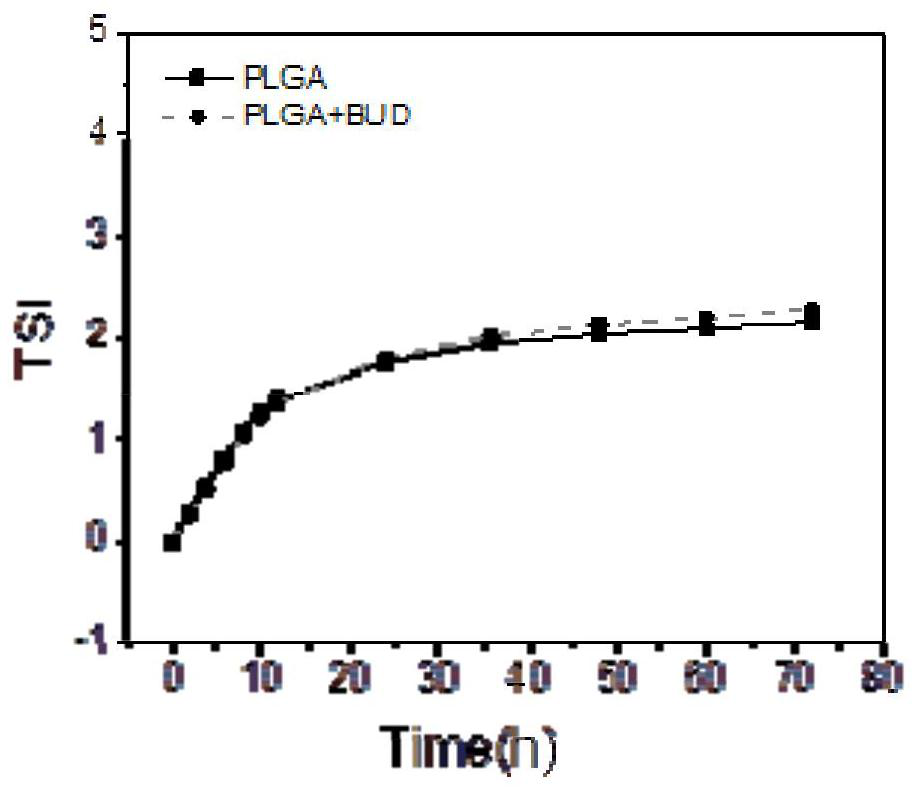
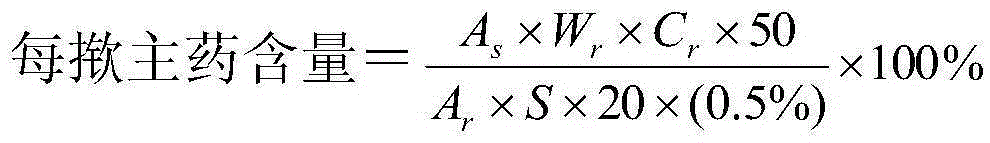Patents
Literature
34 results about "Desonide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a certain skin condition (atopic dermatitis).
Method for treating a respiratory disease
The invention provides a novel method of treating respiratory diseases, e.g., pediatric asthma, in a continuing regimen with not more than one daily dose of the drug budesonide using a nebulizer.
Owner:ASTRAZENECA AB
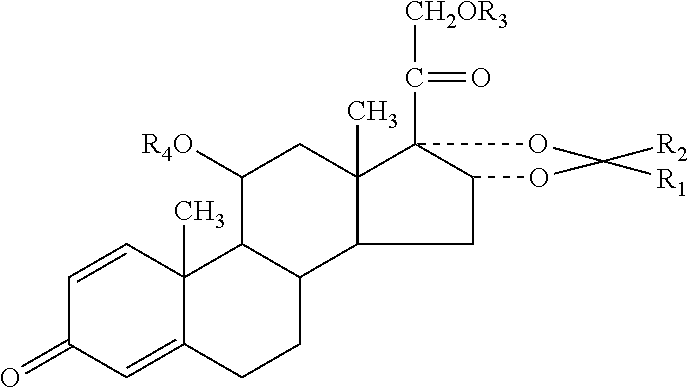
Synthetic method of desonide
The invention discloses a synthetic method of desonide. The synthetic method is characterized by carrying out elimination reaction on prednisone acetate by using sulfur dioxide in the presence of a first catalyst and an inert gas, then carrying out oxidation reaction on a product after elimination by using an oxidant in the presence of a second catalyst, then carrying out condensation treatment on a product after oxidation by using acetone in the presence of a third catalyst, carrying out selective reduction on a product after condensation in the presence of a fourth catalyst and finally carrying out hydrolysis reaction on a product after reduction in the presence of a fifth catalyst, thus preparing desonide. The synthetic method has the beneficial effects that desonide can be obtained by using prednisone acetate as the raw material and carrying out elimination, oxidation, condensation, reduction and hydrolysis; all the solvents in the synthetic process are recycled and reused, heavy metals do not participate in reaction in the reaction process, the technical process is mild and the energy consumption is low, so that the synthetic method is environment-friendly and clean.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD
Desonide cyclodextrin clathrate compound and method for preparing the same
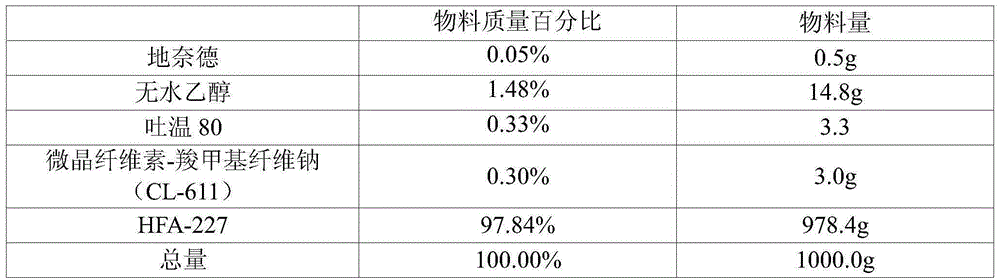
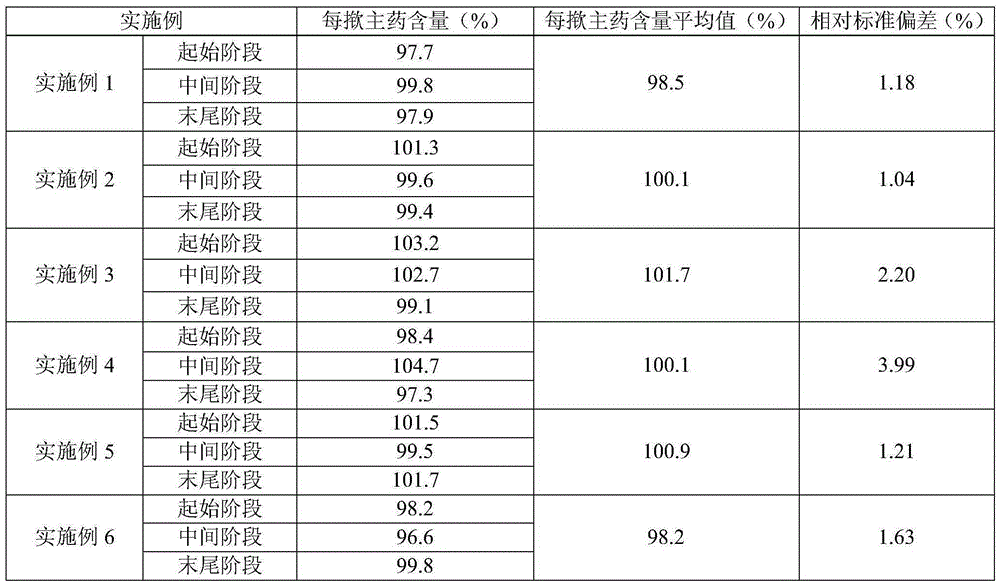
ActiveCN101134108AGood formulation stabilityImprove stabilityOrganic active ingredientsImmunological disordersAlcoholDesonide
The present invention relates to cyclodextrin inclusion compound of desonide and its preparation process. The cyclodextrin inclusion compound of desonide has molecule ratio between desonide and cyclodextrin of 0.1-2. It is prepared through adding the alcohol solution of desonide into the water solution of cyclodextrin to include. Compared with common desonide preparation, the cyclodextrin included desonide preparation has obviously raised stability.
Owner:CHONGQING HUAPONT PHARMA
Combination therapy comprising a thiazole and a corticosteroid to treat skin conditions
InactiveUS20190255023A1Preferable effectReduce inflammation and itchinessOrganic active ingredientsAerosol deliveryFluocinoloneFluocinonide
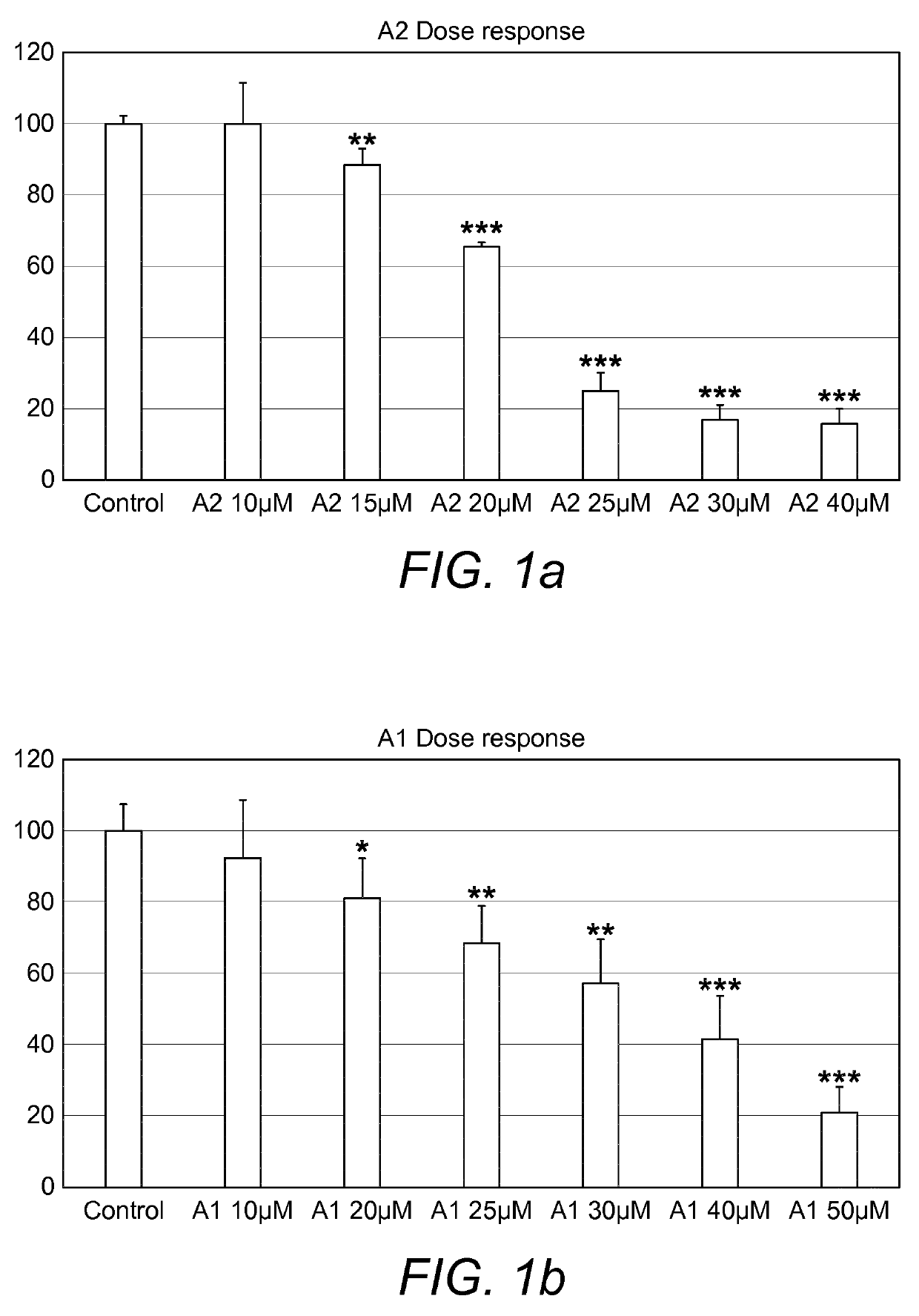
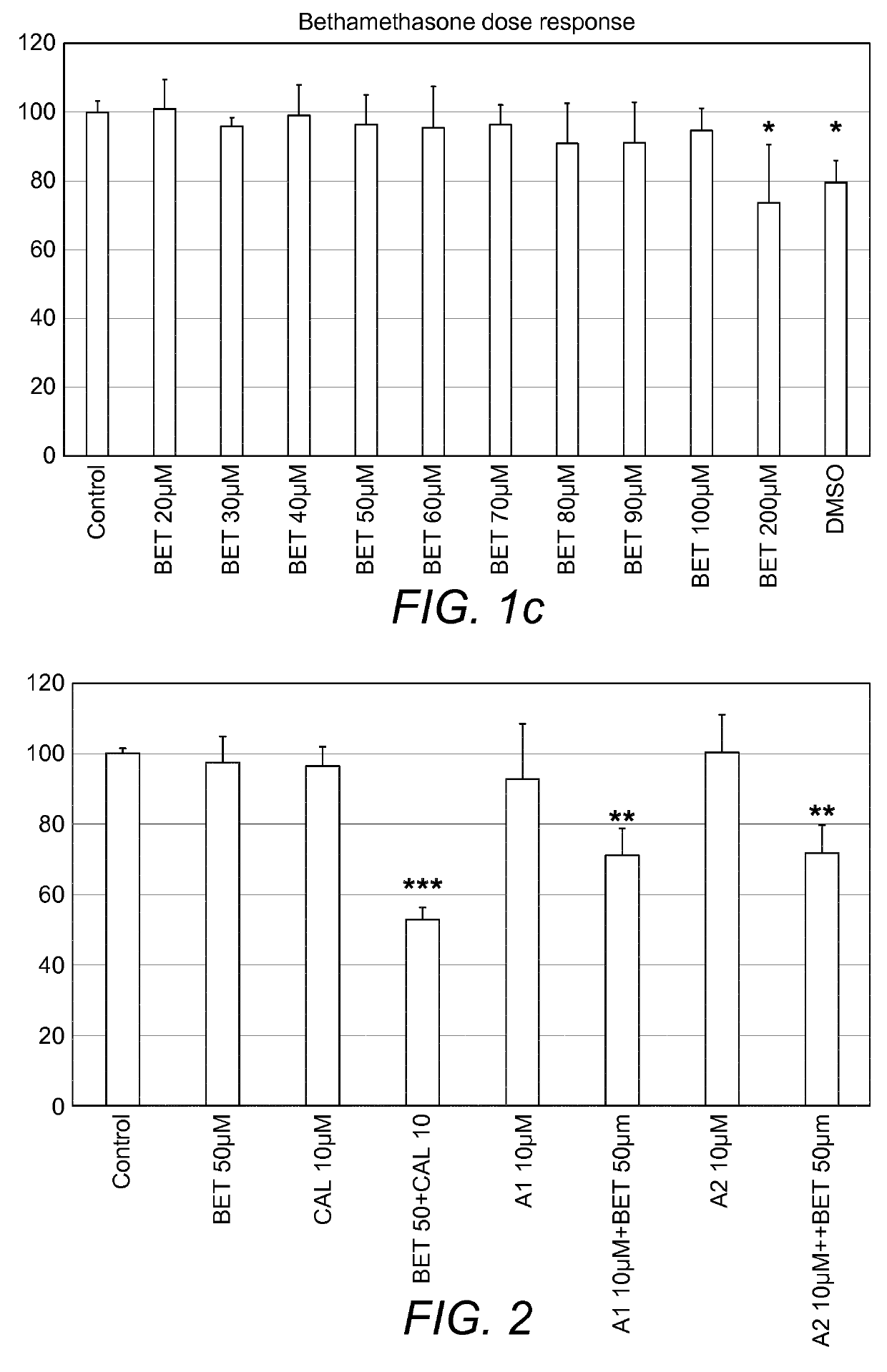
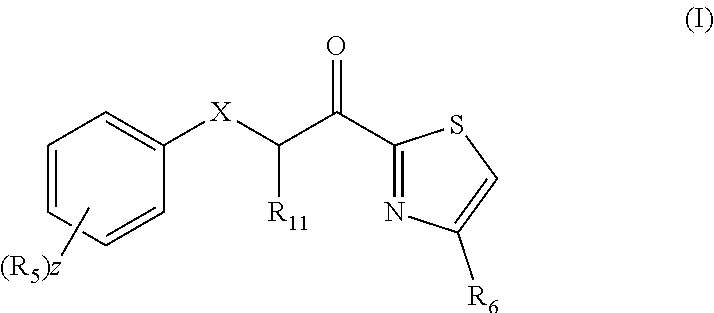
A pharmaceutical composition comprising: (A) at least one compound of formula (I): wherein X is O or S, preferably O R6 is H, C1-6alkyl, —(CH2)pCOOH, —(CH2)pCOOC1-6alkyl, —(CH2)pCONH2, —(CH2)pCONHC1-6alkyl, —(CH2)pCON(C1-6alkyl)2, R11 is H or C1-6 alkyl; each R5 is —OC1-10alkyl, —SC1-10alkyl, —C1-12alkyl, or OAr2; wherein Ar2 is phenyl, optionally substituted with one or more halo; each p is 0 to 3; each z is 1 to 2; or a pharmaceutically acceptable salt, or a hydrate or solvate thereof; and (B) one or more corticosteroid partners, preferably selected from the group consisting of betamethasone, clobetasol, halometasone, dexamethasone, fluocortolone, desoximetasone, diflorasone, fluocinonide, flurandrenolide, halobetasol, amcinonide, halocinonide, triamcinolone, hydrocortisone, aclometasone, fluticasone, mometasone, clocortolone, fluocinolone, desonide, prednisone, prednisolone, and prednicarbate or a pharmaceutically acceptable salt, or a hydrate or solvate thereof, especially betamethasone or a pharmaceutically acceptable salt, or a hydrate or solvate thereof.
Owner:AVEXXIN
Application of compound Haqing injection in preparation of atomization and rectal administration preparation
InactiveCN105311207ASignificant effectSignificant clinical effectOrganic active ingredientsAerosol deliveryDiseaseTracheitis
The invention provides application of a compound Haqing injection in preparation of an atomization and rectal administration preparation, and a preparation method thereof. The preparation can be a drug combination of the compound Haqing injection and one or more of ambroxol, budesonide, terbutaline, dexamethasone and salbutamol, is mainly used for atomization and rectal administration, and has an obvious curative effect on treatment of respiratory diseases such as upper respiratory infection, capillary bronchus and bronchitis.
Owner:耿福能
Method for preparing Desonide cream
InactiveCN107773526AImprove uniformityImprove stabilityOrganic active ingredientsNervous disorderCelsius DegreePreservative
The present invention proposes a method for preparing desonide cream, the method comprising: (1) mixing a consistency regulator, an emulsifier, and a preservative to obtain an oil phase A; (2) mixing purified water, an alkaline regulator, and a stabilizer Mix the enhancers to obtain the water phase B; (3) disperse the desonide in the solvent to obtain the water phase C; (4) mix the oil phase A and the water phase B to obtain the first mixed liquid; (5) Mix the first mixed solution with the aqueous phase C under the condition of greater than or equal to 20 degrees Celsius and lower than 50 degrees Celsius, so as to obtain the desonide cream. The method is simple and convenient to operate, and while the prepared desonide cream has good use performance and curative effect, the high-temperature stability is obviously improved, and the growth of impurities is significantly suppressed.
Owner:HUBEI HUMANWELL CHENGTIAN PHARMA +2
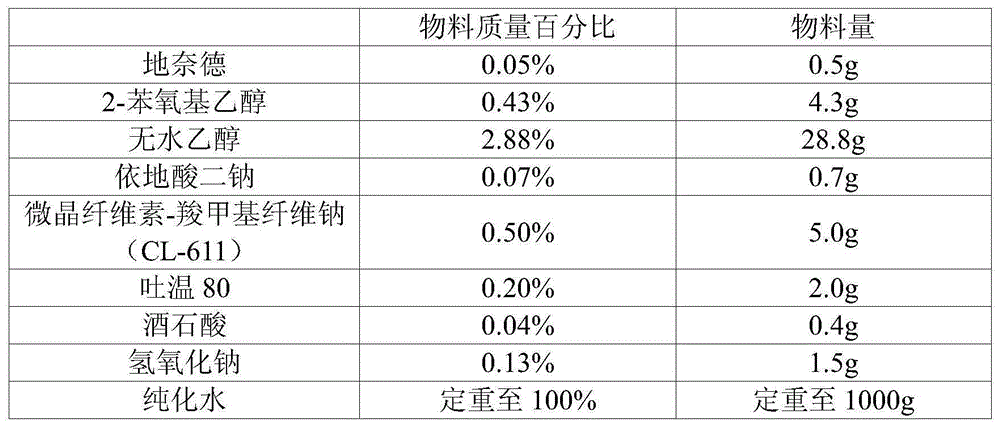
Optical stable desonide preparation
InactiveCN106474047ADetection without interferenceWeakening rangeOrganic active ingredientsAerosol deliveryPharmaceutical formulationPhenylethyl Alcohol
The invention belongs to the field of the pharmaceutical preparation, relates to a desonide preparation, and in particular to a more optical stable desonide preparation which uses phenylethyl alcohol and / or benzyl alcohol and / or 2-phenoxyl ethanol and / or sorbic acid as antiseptics of the desonide preparation to replace the antiseptics methyl-p-hydroxybenzoate and propyl p-hydroxybenzoate. Each component in the desonide preparation does not disturb a related substance detection system. Related substances meet control limitations of related substance guiding principles of chemical medicaments, and photosensitiveness of the preparation is obviously improved. In a contrast investigation of illumination influence factors, the related substance degraded amplitude and the content decrease amplitude in the desonide preparation are all obviously reduced, and the product stability is improved significantly.
Owner:CHONGQING HUAPONT PHARMA
Desonide cyclodextrin clathrate compound and method for preparing the same
ActiveCN101134108BImprove stabilityGood formulation stabilityOrganic active ingredientsPharmaceutical delivery mechanismAlcoholDesonide
The present invention relates to cyclodextrin inclusion compound of desonide and its preparation process. The cyclodextrin inclusion compound of desonide has molecule ratio between desonide and cyclodextrin of 0.1-2. It is prepared through adding the alcohol solution of desonide into the water solution of cyclodextrin to include. Compared with common desonide preparation, the cyclodextrin includeddesonide preparation has obviously raised stability.
Owner:CHONGQING HUAPONT PHARMA
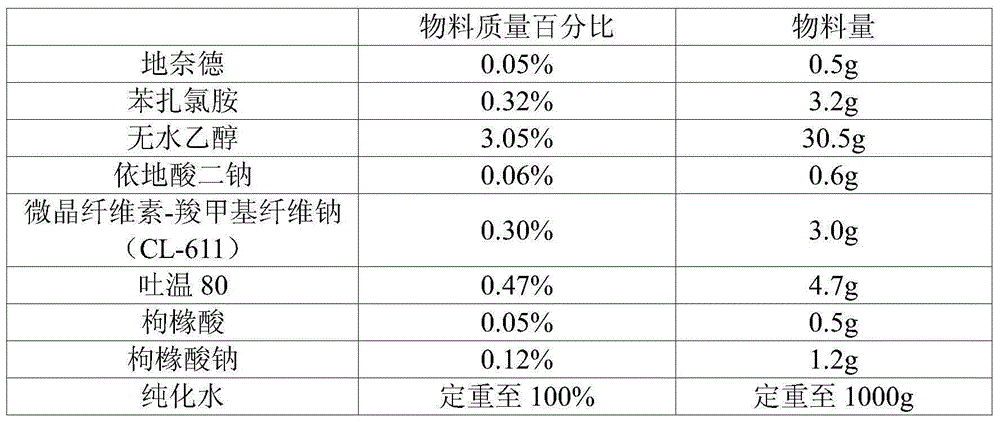
Topical spraying agent containing desonide
InactiveCN106474062AImprove complianceExpand the spraying areaOrganic active ingredientsAerosol deliveryMedicineTopical spray
The invention belongs to the field of pharmaceutical preparations, relates to a pharmaceutical preparation containing desonide, and specifically relates to a topical spraying agent containing the desonide. A drug contained in the topical desonide spraying agent is a stable-quality desonide drug containing desonide micro powder, and the desonide drug comprises, by weight, 0.05%-0.2% of the desonide micro powder and balance of pharmaceutically acceptable excipients. The topical desonide spraying agent is sprayed directly on a site of action, and is uniform to distribute, the effect is fast to take, in addition, by use of a direct spray method, contact with microorganism is reduced, by use of a closed dark storage container, contact with water and oxygen in the air can be avoided, and the drug stability can be improved.
Owner:CHONGQING HUAPONT PHARMA
Antifungal formulations
A topical composition and the method using the composition, which contains an antifungal agent and a low potency anti-inflammatory steroid which is safe and effective such as desonide or its derivative. The low potency steroid agent does not cause side effects such as skin atrophy, striae and hypopigmentation. The composition can be formulated in a dosage form such as a cream, ointment, gel, lotion, foam, powder, aerosol, spray, shampoo, or liquid solution. The composition can be used to treat a fungal disease such as tinea pedis, tinea capitis, tinea corporis, tinea versicolor, tinea cruris, and candidiasis as well as intertriginous dermatitis complicated by candidiasis.
Owner:GOLDSTEIN JAY A +2
Desonide lotion with more stable quality
InactiveCN106474059AImprove quality stabilityImprove the defects of obvious settlement and uneven contentOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseSynthetic Polymeric Macromolecules
The invention relates to a desonide preparation, specifically to a desonide lotion with more stable quality, belonging to the field of medicinal preparations. The desonide lotion with more stable quality is a lotion containing a stable matrix which is one or more selected from a group consisting of a natural high polymer or a derivative thereof, a cellulose ether compound and a synthetic polymeric compound. The desonide lotion has good physical stability and chemical stability; the physical stability is embodied in that active components are suspended in the lotion and free of obvious settlement trend, and the desonide lotion has good content uniformity and does not need shaking in use, which greatly facilitates usage of the desonide lotion by patients, especially elder patients; and the chemical stability is embodied in that related substances in the lotion accord with control limits prescribed in guidance principles for related substances of chemical drugs, and components in the prescription of the desonide lotion pose no interference to a detection system for the related substances, which is better than commercially available products at present.
Owner:CHONGQING HUAPONT PHARMA
Method for synthesizing desonide impurity
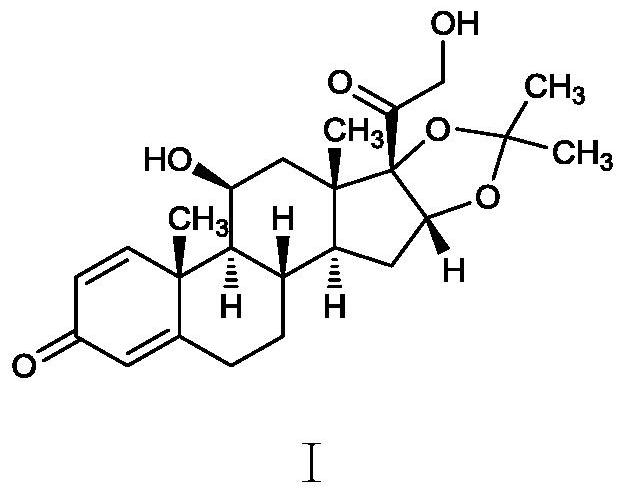
The invention discloses a method for synthesizing desonide impurity 11 [beta], 16 [alpha], 17 [alpha]-trihydroxypregna-1, 4-diene-3, 20-dione-21 aldehyde-16, 17-acetal acetone. The method comprises the following steps: taking desonide as an initial raw material, catalyzing by using anhydrous cupric sulfate, introducing air to oxidize, carrying out hydrolysis reaction under the catalysis of acid, and drying by using sodium sulfate to obtain the desonide impurity 11 [beta], 16 [alpha], 17 [alpha]-trihydroxypregna-1, 4-diene-3, 20-dione-21 aldehyde-16, 17-acetal acetone. The synthesis route is short, the reaction condition is mild, the impurity can be obtained without column chromatography, and the purity of the obtained impurity is 97% or above.
Owner:CHONGQING HUABANGSHENGKAI PHARM
External aerosol containing desonide
InactiveCN106474063AImprove complianceExpand the spraying areaOrganic active ingredientsNervous disorderMicroorganismMedicine
The invention belongs to the field of drug preparations and relates to a drug preparation containing desonide, in particular to an external aerosol containing desonide. A content drug of the desonide aerosol is a desonide drug stable in quality and containing desonide micropowder. The desonide aerosol consists of the following components in percentage by weight: 0.05-0.2% of the desonide micropowder and the balance of pharmaceutically acceptable auxiliary materials. The desonide aerosol is directly sprayed to an action part, is distributed uniformly and quickly takes effect, and due to the use method of direct spraying, the contact with microorganisms is reduced; and a closed lightproof storage container avoids contact with water and oxygen in air, so that the drug stability is improved.
Owner:CHONGQING HUAPONT PHARMA
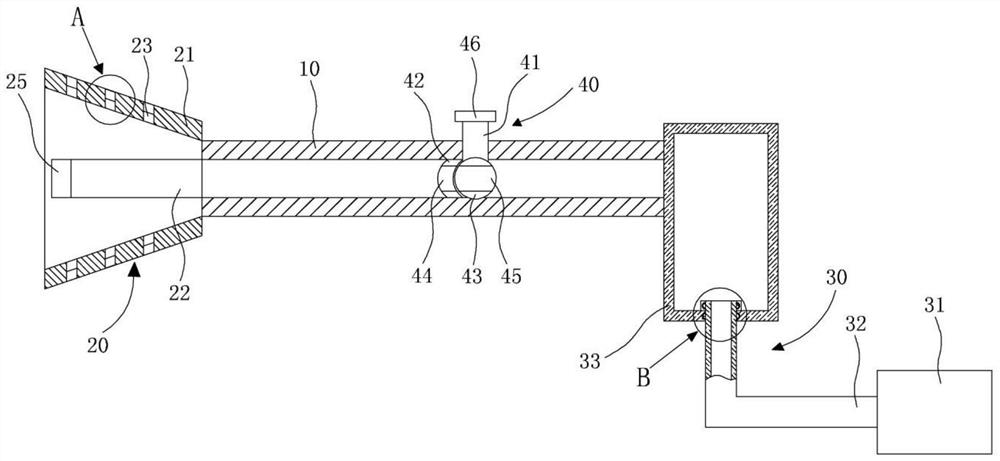
Budesonide atomization spraying device
The invention discloses a budesonide atomization spraying device. The budesonide atomization spraying device comprises a spraying main pipe, a spraying head and an atomization assembly; one end of thespraying main pipe is detachably connected with the spraying head, and the other end of the spraying main pipe is connected with the atomization assembly; the spraying head comprises a spraying coverand a spraying branch pipe; one end of the spraying cover is in threaded connection with the outer side face of the spraying main pipe, and the other end of the spraying cover covers lips of a patient; and one end of the spraying branch pipe is inserted into the inner wall of the spraying main pipe, and the other end of the spraying branch pipe is inserted into the mouth of the patient. Accordingto the budesonide atomization spraying device, atomized liquid medicine sprayed out of the spraying main pipe is limited in the spraying cover through the spraying head, and meanwhile, the atomized liquid medicine is directly sprayed into the mouth of the patient through the spraying branch pipe, and therefore, waste of the medicine and spraying of the medicine to the face of the patient are avoided.
Owner:重庆市公共卫生医疗救治中心
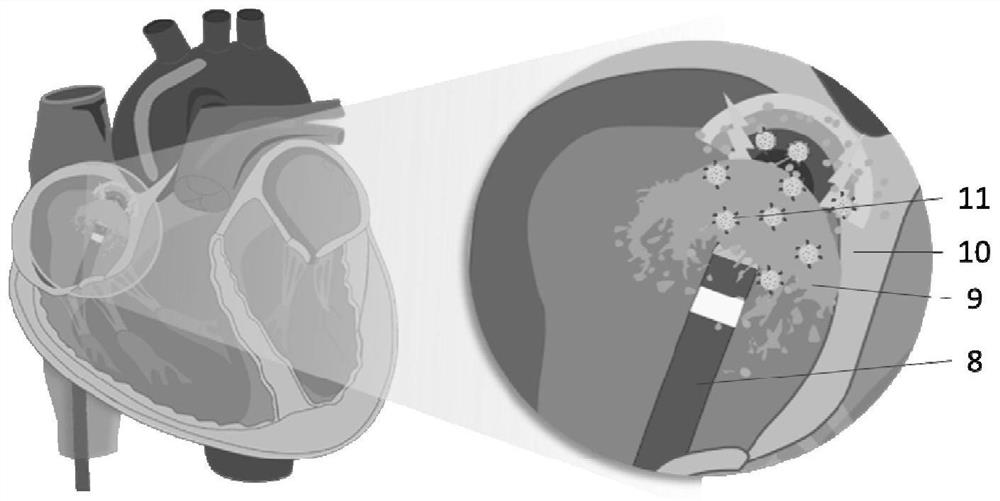
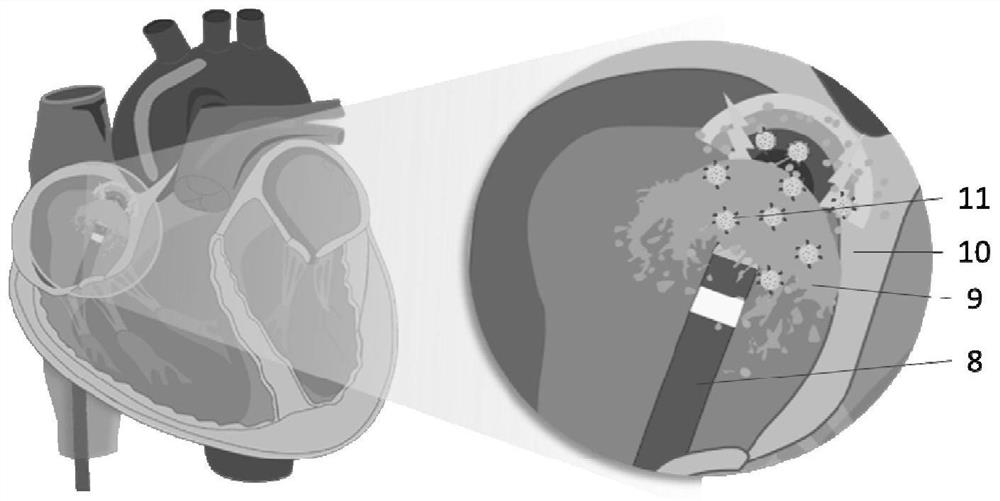
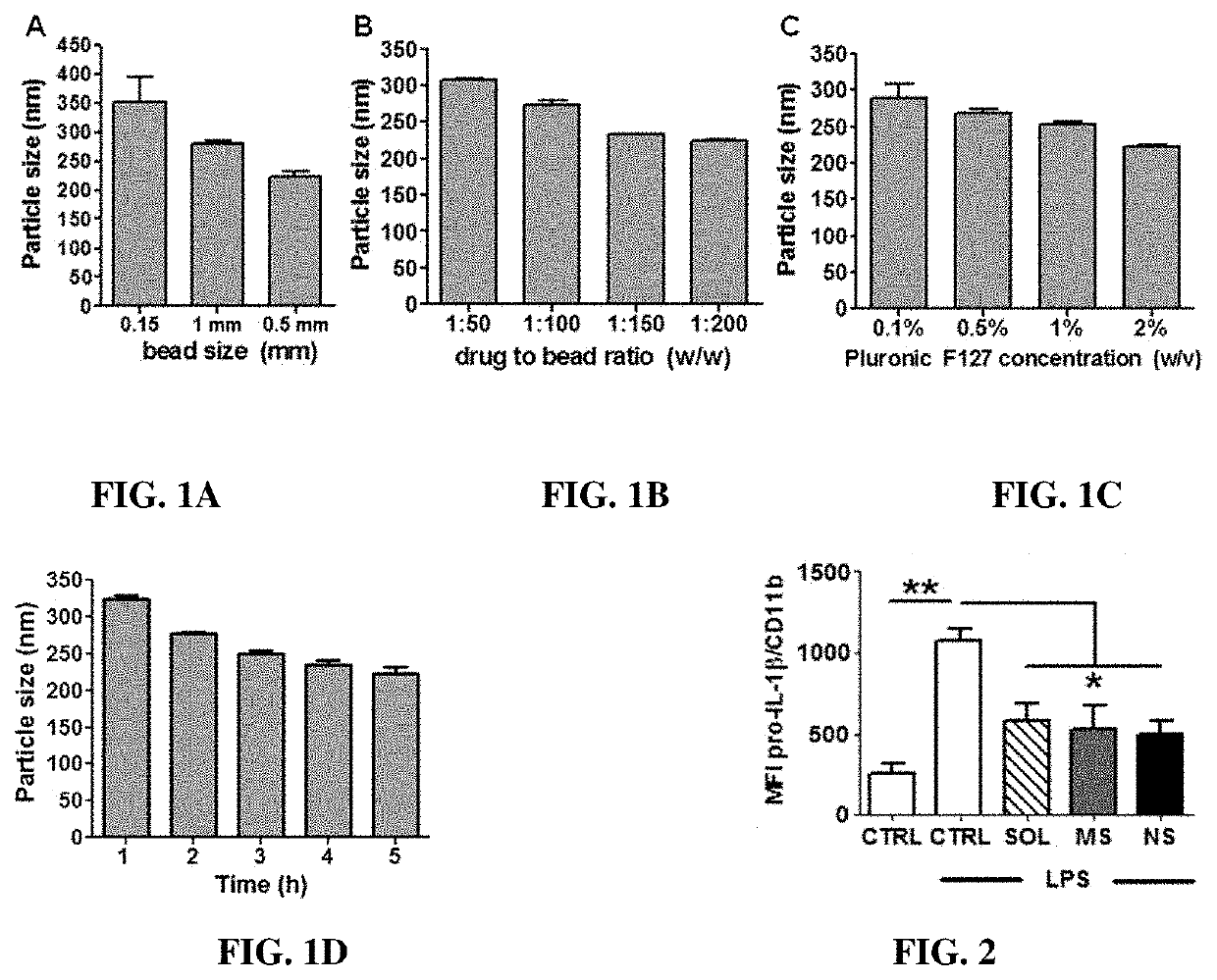
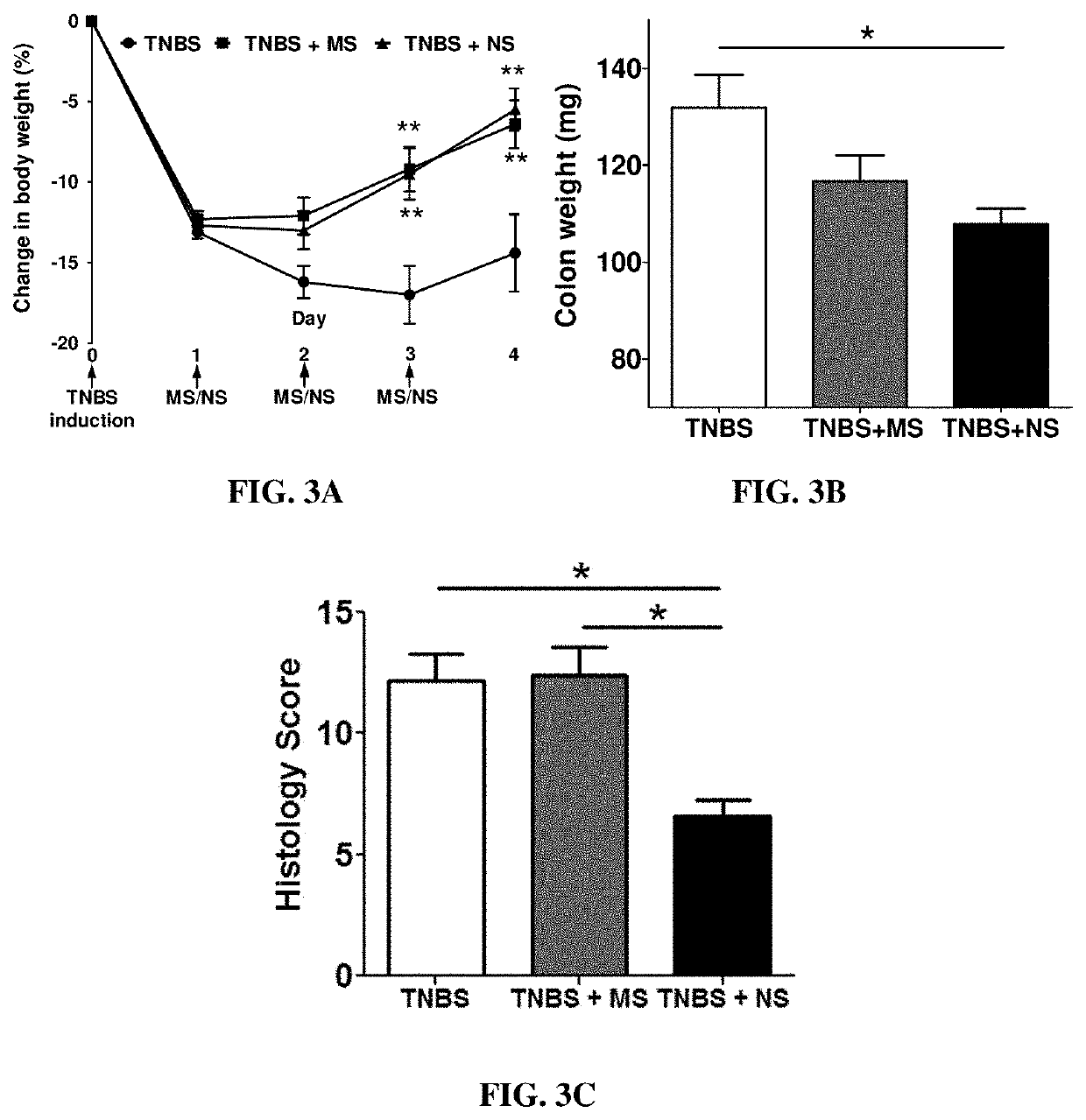
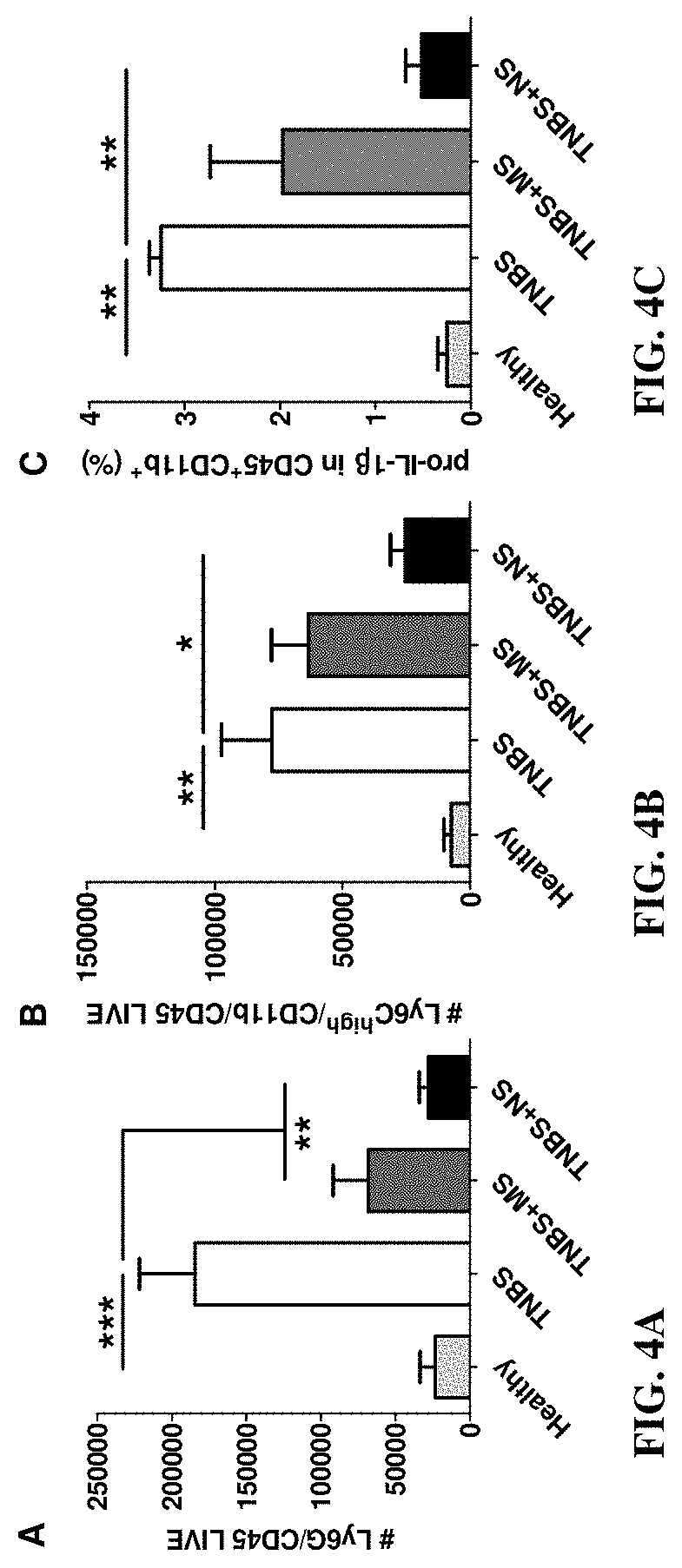
Nano-preparation capable of being locally delivered and used for inhibiting radiofrequency ablation cardiac tissue inflammation and preparation method and application of nano-preparation
ActiveCN111617034AStrong local anti-inflammatory effectLess systemic reactionOrganic active ingredientsMedical devicesGlucocorticoidBudesonide
The invention belongs to the field of nano-pharmaceutical preparations, and particularly relates to a nano-preparation capable of being locally delivered and used for inhibiting radiofrequency ablation cardiac tissue inflammation and a preparation method and application of the nano-preparation. The nano-preparation comprises a nano-carrier and fat-soluble glucocorticoid encapsulated in the nano-carrier, and the fat-soluble glucocorticoid is included in the nano-preparation in a therapeutically effective amount, so that the nano-preparation can inhibit radiofrequency ablation cardiac tissue inflammation. PLGA-BUD-CS nanoparticles provided by the preferred embodiment of the invention can stably exist in water to form a uniform suspension, local delivery of the inner wall of the heart cavitycan be achieved through a radiofrequency ablation catheter, the fat solubility of budesonide ensures that enough medicine enters local tissue, the anti-inflammatory effect is achieved, and moreover, by PLGA, the medicine stays in the tissue for a longer time.
Owner:胥玲玲
Methods for treating and preventing symptoms of asthma with a corticosteroid pharmaceutical composition
PendingCN112997256AOrganic active ingredientsDigital data information retrievalFluticasone propionateMometasone furoate
A method is provided for treating or preventing symptoms of asthma in a subject in need thereof by administering a corticosteroid pharmaceutical composition to a subject qualified for over-the-counter access to the corticosteroid pharmaceutical composition. In some embodiments, the corticosteroid pharmaceutical composition includes a class B corticosteroid, a glucocorticosteroid, budesonide, ciclesonide, fluticasone furoate, mometasone furoate, fluticasone propionate, or beclomethasone dipropionate.
Enema for rectal application
PendingUS20220142921A1Improve curing effectPrevent relapseOrganic active ingredientsAerosol deliveryUlcerative colitisBowels diseases
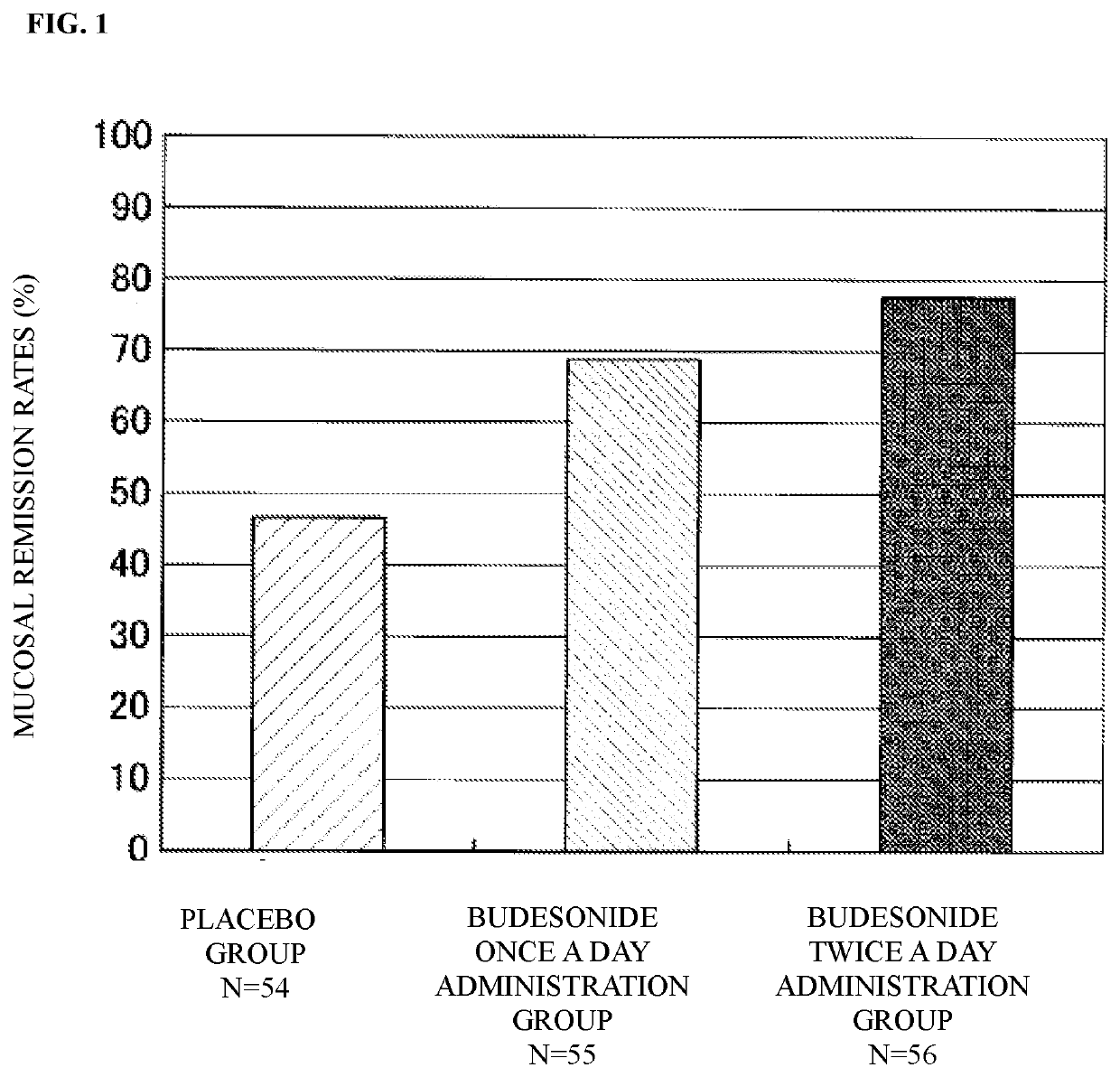
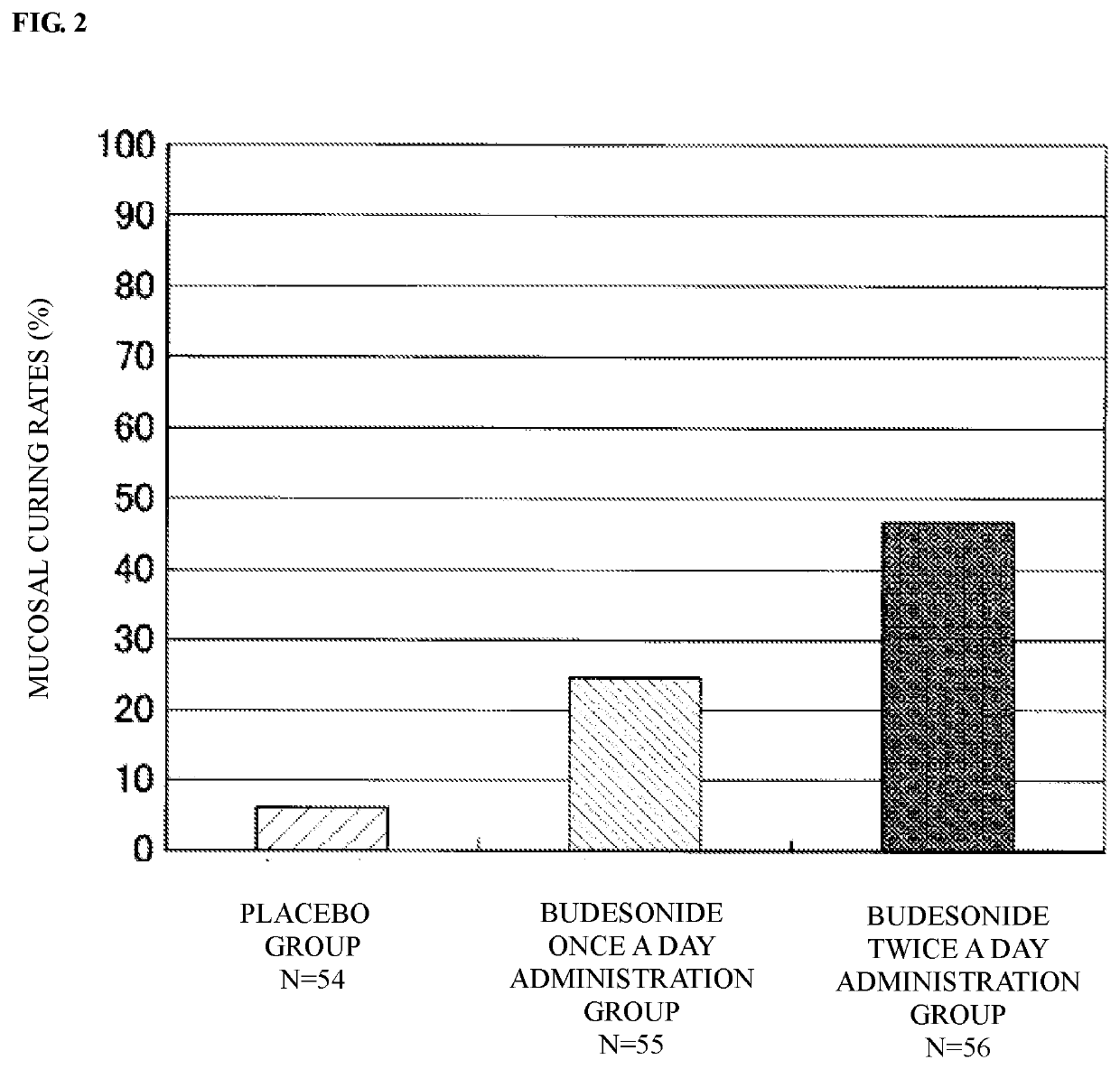
Provided is an enema for rectal application containing budesonide as an active ingredient in order to treat inflammatory bowel disease, or to prevent a relapse. The enema for rectal application containing budesonide as the active ingredient, in which 1.5 to 2.5 mg of budesonide per dose is administered twice a day for 6 weeks in order to treat inflammatory bowel disease, or to prevent a relapse; the enema for rectal application described above, in which a dose of budesonide is 2.0 mg per dose; the enema for rectal application according to any one of the above, which is taken in order to treat ulcerative colitis or Crohn's disease, or to prevent a relapse; the enema for rectal application according to any one of the above, which has a foamy shape or a liquid shape.
Owner:DR FALK PHARMA GMBH
A kind of medicine for treating children's bronchial asthma and preparation method thereof
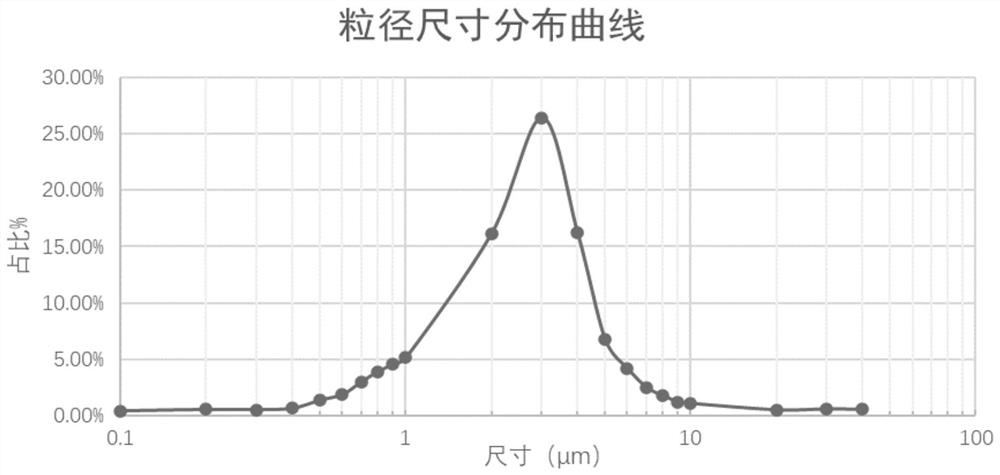
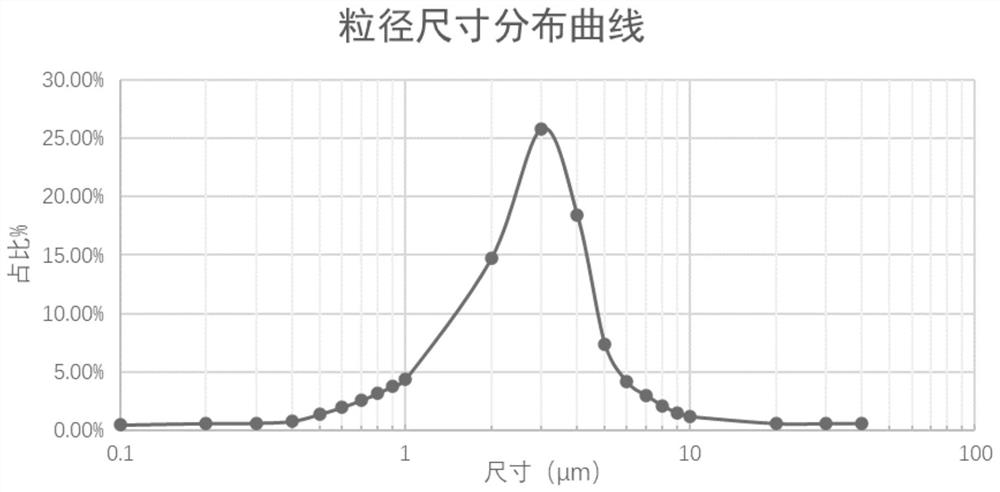
ActiveCN112274497BImprove stabilityUniform particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsFormularySalbutamol
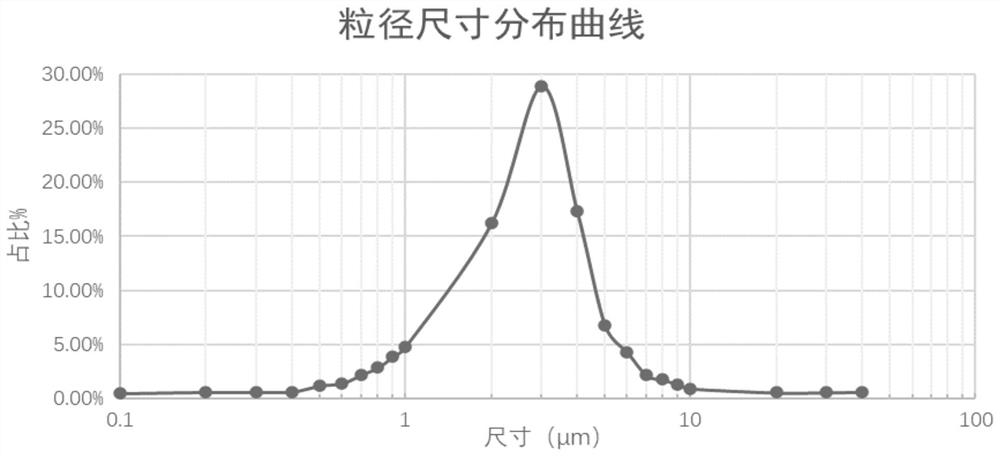
The invention relates to a medicine for treating children's bronchial asthma, which is characterized in that it contains salbutamol, budesonide and soybean lecithin. In the present invention, by rationally designing the formula, the medicine for treating children's bronchial asthma has the advantages of good stability and uniform particle size, and the particle size distribution and average particle size meet the requirements of lung inhalation preparations.
Owner:QINGDAO CENT HOSPITAL
A kind of moxifloxacin hydrochloride ear drops and preparation method thereof
ActiveCN104224802BBroad spectrum antibacterialStrong antibacterial effectAntibacterial agentsOrganic active ingredientsPolyethylene glycolEar drop
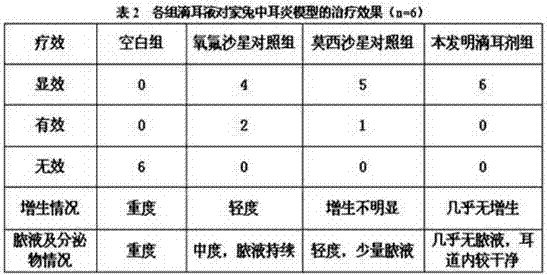
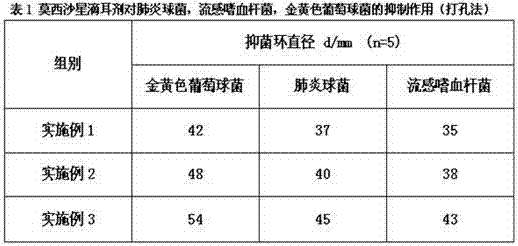
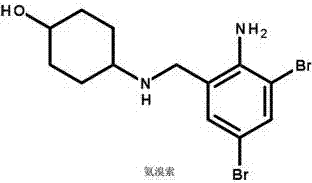
The invention discloses moxifloxacin hydrochloride in-situ gel ear drops. The ear drops are prepared from components such as moxifloxacin hydrochloride used as a main medicine ingredient, budesonide and ambroxol which are used as auxiliary medicines as well as carbomer, polyethylene glycol, menthol, borneol, boric acid and the like which are additionally added. The invention further discloses a preparation method of the moxifloxacin hydrochloride in-situ gel ear drops. The moxifloxacin hydrochloride is a broad-spectrum antibiotic of the fourth generation of quinolones, can perform sterilization effectively and is lasting in effect; the gel ear drops can act on affected parts directly, and the dosage is reduced, so that adverse reactions caused by whole body absorption are reduced, and the patient compliance is improved. The budesonide and the ambroxol are combined for use, so that inflammatory reactions and immunity suppression effects of mucosae can be reduced, discharge of otitis media hydrops and secretions is promoted, and the synergistic effect of eliminating etiologies and restoring functions is realized.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
Preparation method of oxidation impurity
The invention belongs to the technical field of medicine synthesis, and particularly relates to a preparation method of an oxidation impurity. The oxidation impurity is 11beta, 16alpha, 17alpha-trihydroxy-3-oxoandrostane-1, 4-diene-17-carboxylic acid ring 16, 17-acetal acetone, and the method comprises the following steps: taking desonide as a starting raw material, and introducing air to oxidize in the presence of inorganic alkali to obtain the oxidation impurity. The synthesis route is short, raw materials are easy to obtain, operation is simple, reaction conditions are mild, the impurity can be obtained without column chromatography, and the purity of the obtained impurity is 97% or above.
Owner:CHONGQING HUABANGSHENGKAI PHARM
Co-amorphous powder for treating asthmatic lung diseases and preparation method thereof
ActiveCN110025578BImprove solubilityImprove stabilityPowder deliveryOrganic active ingredientsDiseaseSalbutamol
The invention discloses a co-amorphous powder for treating asthmatic pulmonary diseases and a preparation method thereof. Dissolving salbutamol, poorly soluble corticosteroid and carrier in a solvent, and then spray-drying to form a co-amorphous form among the three components to obtain a co-amorphous powder for treating asthmatic lung diseases. The present invention is based on the physical and chemical properties of salbutamol and the insoluble corticosteroid budesonide. A large number of experimental studies have been carried out, and a ternary co-amorphous substance containing three components of salbutamol, budesonide and serine has been found, which can not only significantly improve the Stability, but also effectively increase the solubility of the corticosteroid budesonide. In addition, serine is easily soluble in water and forms a colloid in 90%-95% (v / v) ethanol, which can evenly disperse salbutamol and insoluble corticosteroid budesonide, so that the final spray-dried amorphous powder has a uniform particle size.
Owner:SHENZHEN NYCRIST TECH CO LTD
A locally deliverable nano-preparation for inhibiting inflammation of radiofrequency ablation cardiac tissue, its preparation method and application
ActiveCN111617034BHas a broad-spectrum antibacterial effectPrevent edemaOrganic active ingredientsMedical devicesNanocarriersGlucocorticoid
The invention belongs to the field of nano-pharmaceutical preparations, and in particular relates to a locally-deliverable nano-preparation for inhibiting radiofrequency ablation of heart tissue inflammation, a preparation method and application thereof. The nano-preparation includes a nano-carrier and a fat-soluble glucocorticoid contained in the nano-carrier, and the fat-soluble glucocorticoid is included in the nano-preparation in a therapeutically effective amount, so that the nano-preparation can suppress radio frequency Ablation of inflamed heart tissue. The PLGA-BUD-CS nanoparticles provided by the preferred example of the present invention can exist stably in water, form a uniform suspension, and can be locally delivered to the inner wall of the heart cavity through a radiofrequency ablation catheter, and the fat solubility of budesonide ensures sufficient drug entry Local tissues, play an anti-inflammatory effect, and PLGA makes the drug stay in the tissue for a longer time.
Owner:胥玲玲
Mucus-penetrating budesonide nanosuspension enema for local treatment of inflammatory bowel disease
InactiveUS20200306202A1Reduce deliveryImprove permeabilityPowder deliveryOrganic active ingredientsNanoparticleBowels diseases
Inflammatory bowel disease (IBD) is a chronic inflammatory gastrointestinal disorder that affects more than 1 million individuals in the USA. Described herein are nanoparticle mucus penetrating formulations for administration of drugs for improved mucosal distribution and tissue penetration such as in the treatment of IBD.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
A kind of preparation method of budesonide sterile raw material and its suspension for inhalation
ActiveCN108175763BStability is not affectedPharmacochemical properties did not changeOrganic active ingredientsDispersion deliveryPharmaceutical drugWater insoluble drug
The invention provides a preparation method of a budesonide sterile material and suspension thereof for atomization inhalation. On one hand, the problem of instability generated when dry heat sterilization adopts water insoluble medicine-the budesonide sterile material of which the sterilization temperature is higher than 160 DEG C can be solved, and on the other hand, the problem of low sterile guarantee when the water insoluble medicine-the budesonide sterile raw material adopts dry heat sterilization of 100-130 DEG C is solved. Therefore, the method is especially suitable for preparing thebudesonide suspension used for atomization inhalation.
Owner:苏州西克罗制药有限公司
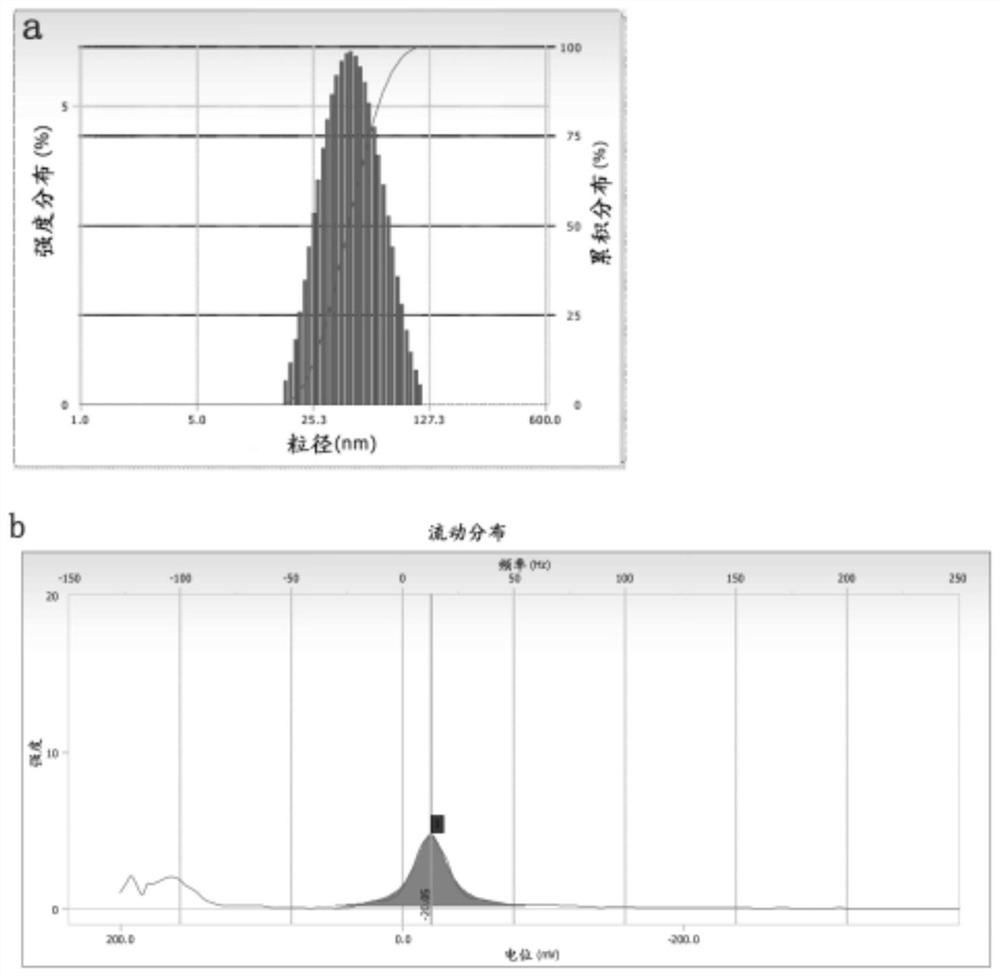
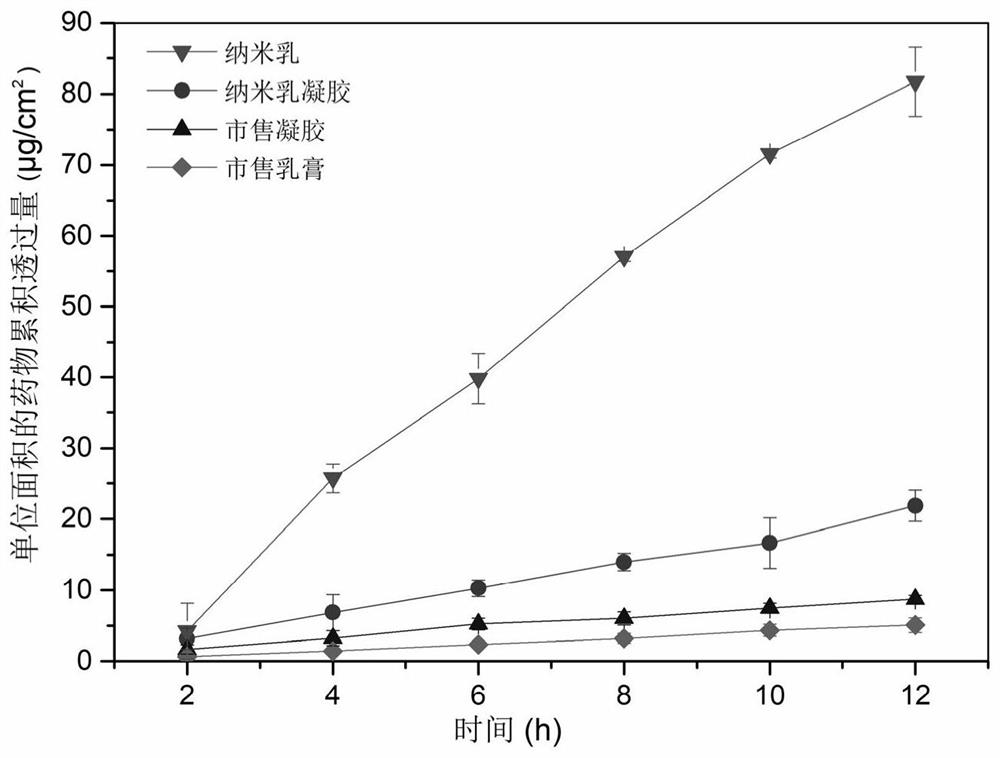
Desonide nano-emulsion gel composition and preparation method thereof
PendingCN112263542AImprove solubilityImprove stabilityOrganic active ingredientsAntipyreticTreatment effectOil phase
The invention provides a desonide nano-emulsion gel composition and a preparation method thereof. The desonide nano-emulsion gel composition comprises the following components in percentage by weight:0.05%-1.0% of desonide, 5%-20% of an oil phase component, 0.1%-10% of an emulsifier, 0.5%-9% of a co-emulsifier, 0.5%-10% of a wetting agent, 0.5%-3% of a gel matrix, 3%-10% of a humectant, 0.001%-0.1% of a metal ion chelating agent, 0.001%-0.2% of a preservative, 0.01%-0.5% of an antioxidant, and 89.388% to 90.338% of water. The oil-in-water desonide nano-emulsion gel composition provided by theinvention has good stability, adhesion and spreadability, can improve the penetration capacity and targeting effect of the desonide on the cuticle of the skin and improve the transdermal absorption of the desonide, and has strong penetration capacity, viscosity suitable for local administration and long drug release time, and the treatment effect of the desonide is improved.
Owner:BRIGHT FUTURE PHARMA LAB LTD (CN)
Montelukast esters and pharmaceutical compositions containing the same
The present invention relates to esters of Montelukast with corticosteroids, preferably with des-ciclesonide, triamcinolone, budesonide or flunisolide, pharmaceutical compositions containing the same and the use thereof in the treatment of several respiratory tract diseases, especially asthma, chronic obstructive pulmonary disease (COPD) and allergic rhinitis. The invention further relates to the process for preparing said esters.
Owner:GENETIC SPA
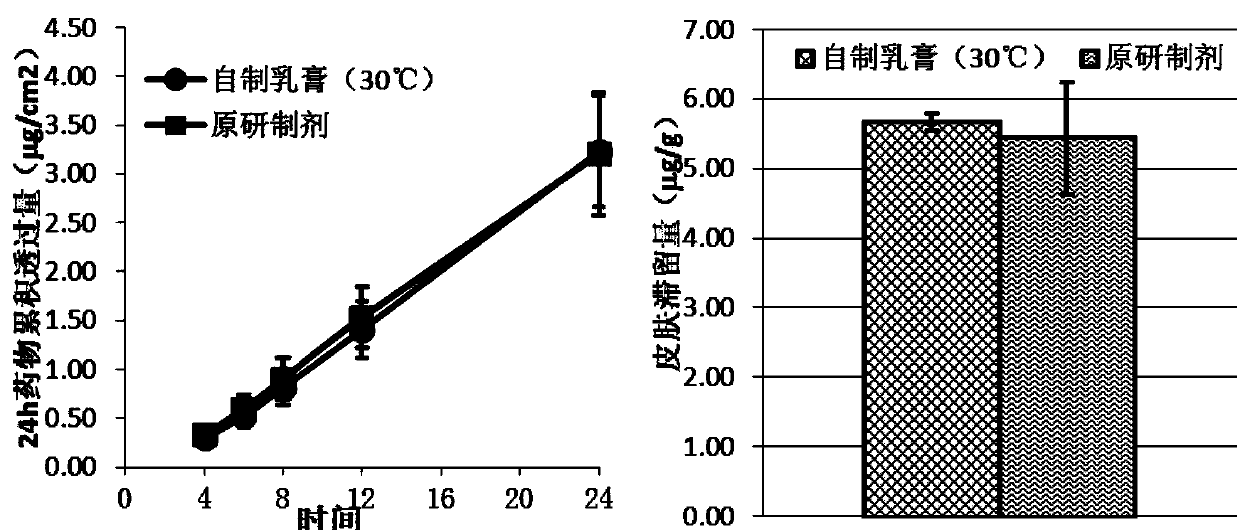
Desonide cream and preparation method thereof
ActiveCN107260656BImprove high temperature stabilityInhibit growthOrganic active ingredientsAerosol deliveryBiochemistryBULK ACTIVE INGREDIENT
Owner:HUBEI HUMANWELL CHENGTIAN PHARMA +2
Nanoliposomes comprising corticosteroid as medicaments and methods to prepare them
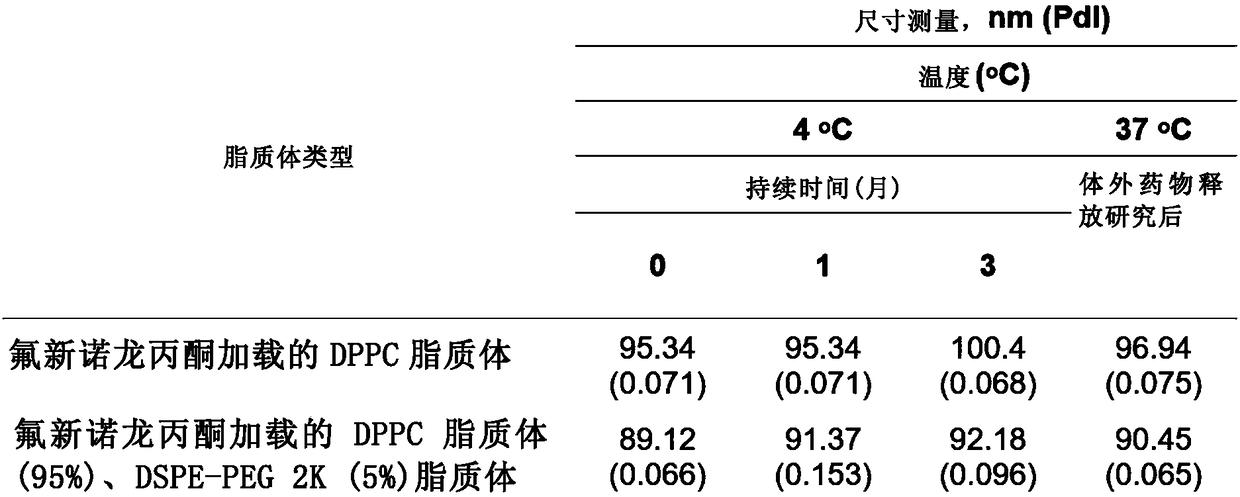
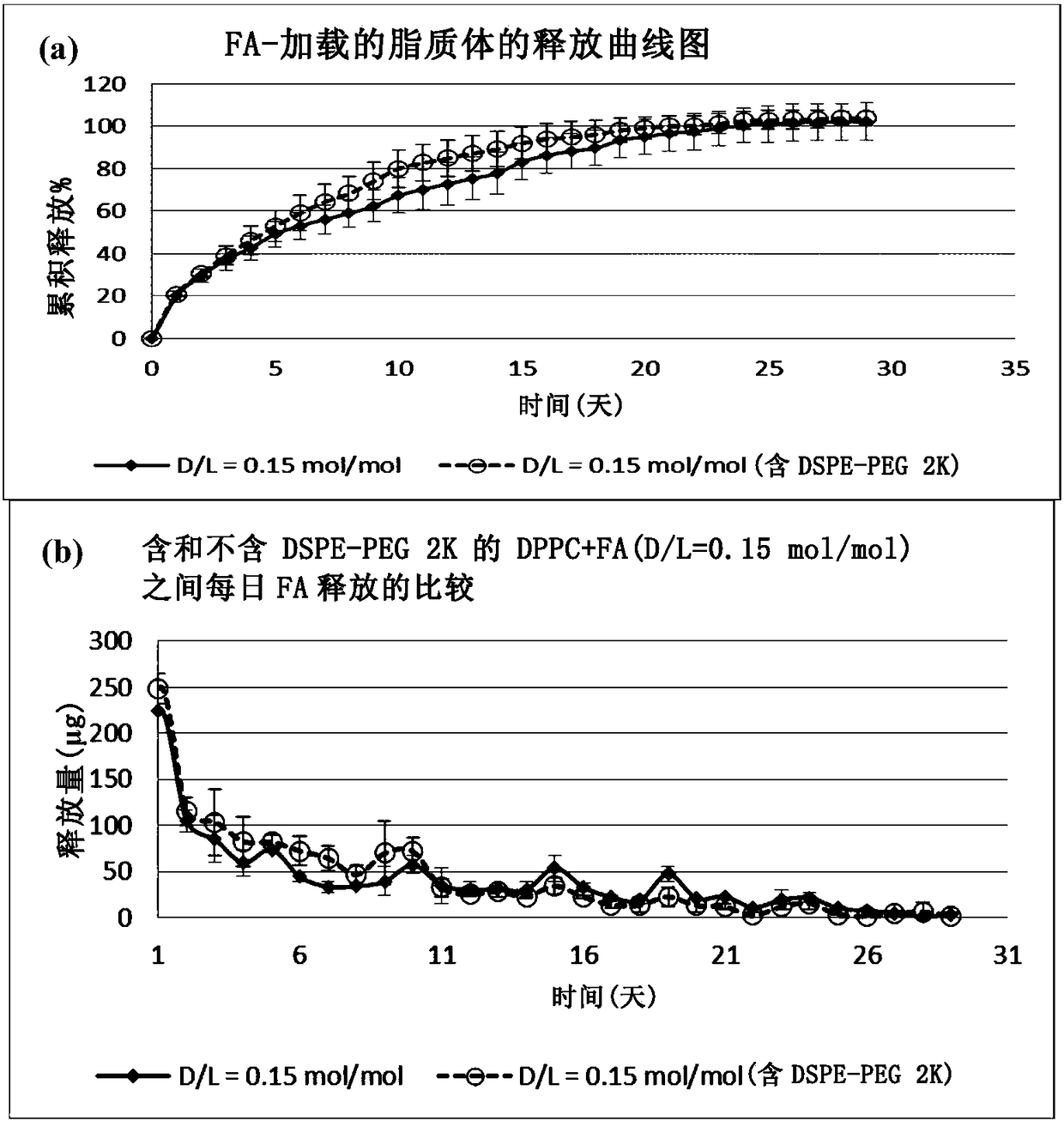
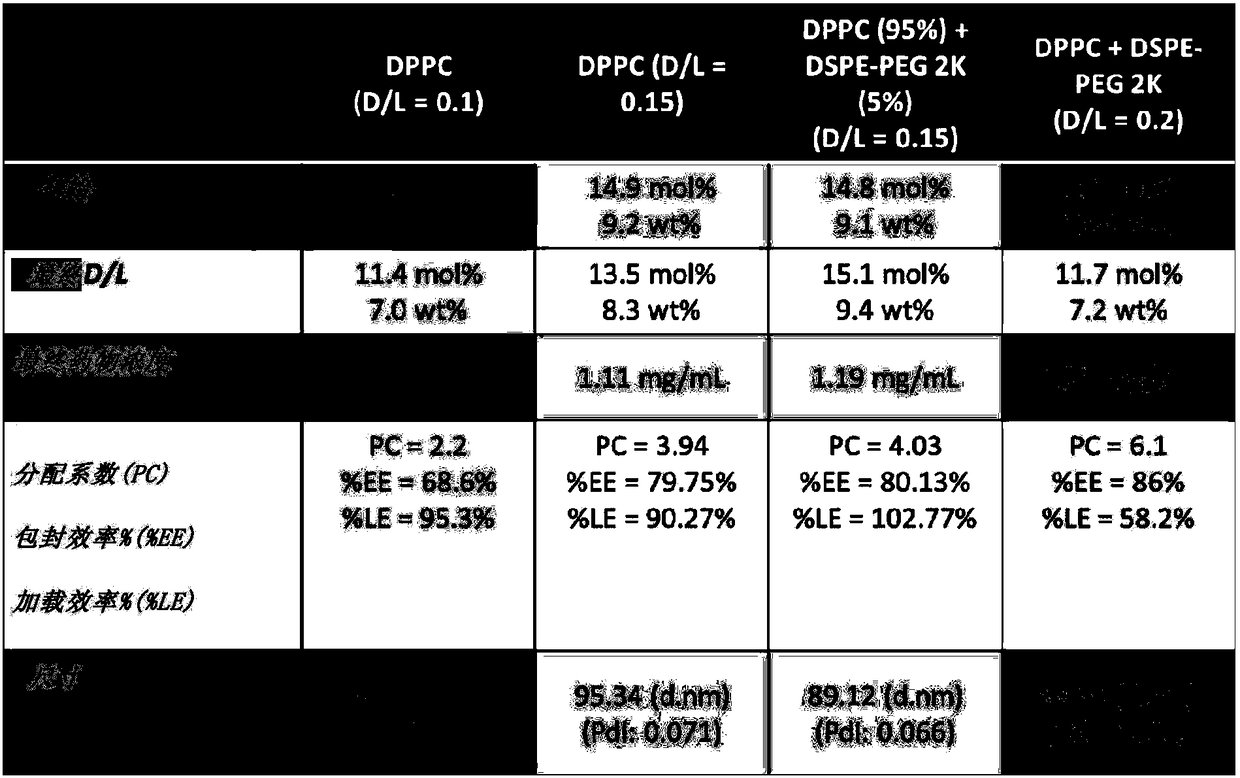
This invention relates to a nanoliposome comprising at least one outer lipid bilayer and at least one corticosteroid encapsulated bythe at leastone lipid bilayer, wherein the ratio of the corticosteroid and lipids forming the lipid bilayer is preferably between 0.01 - 0.5, 0.1 - 0.3 or 0.12 - 0.18. The corticosteroid may be a group B corticosteroid, including triamcinolone acetonide, fluocinoloneacetonide, triamcinolone alcohol, mometasone, amcinonide, budesonide, desonide, fluocinonide and halcinonide. Preferably, the size of the nanoliposome is between 1 0 - 1 000 nm or 50 - 150 nm. The present invention also relates to the use of the nanoliposome of the invention for use as a medicam ent and for use in the treatment of a cardiovascular disease. Further, the invention is directed to a method to prepare the nanoliposome of the invention, which may further include an extruding step.
Owner:NANYANG TECH UNIV
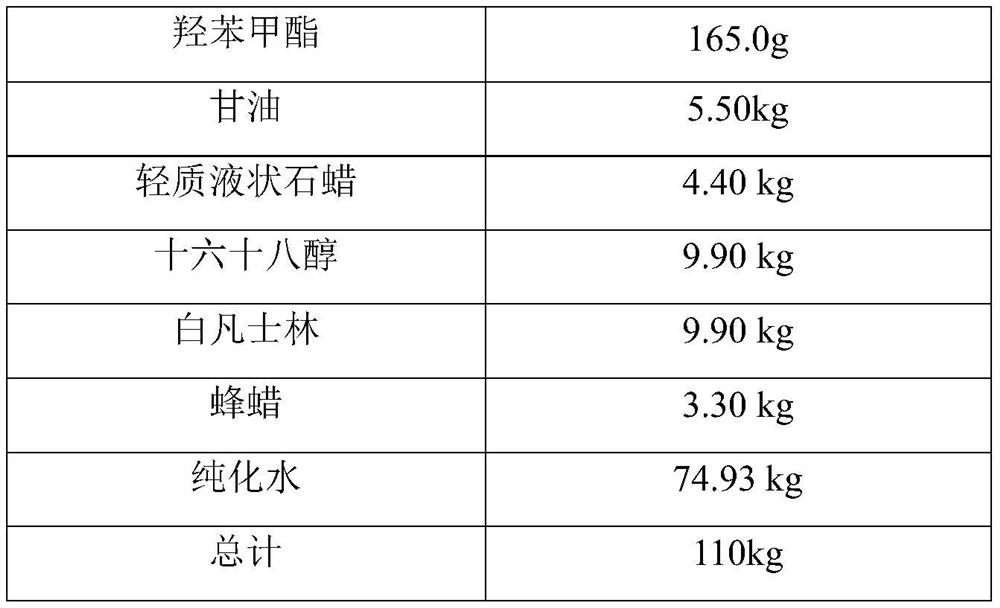
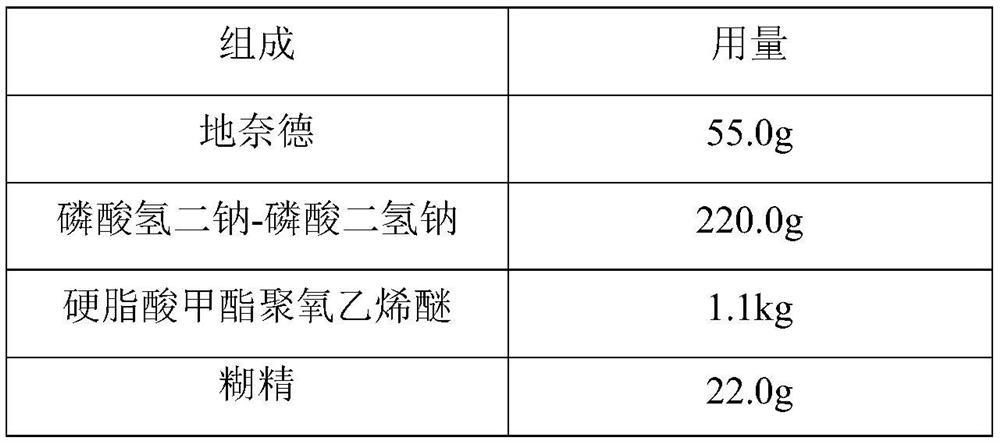
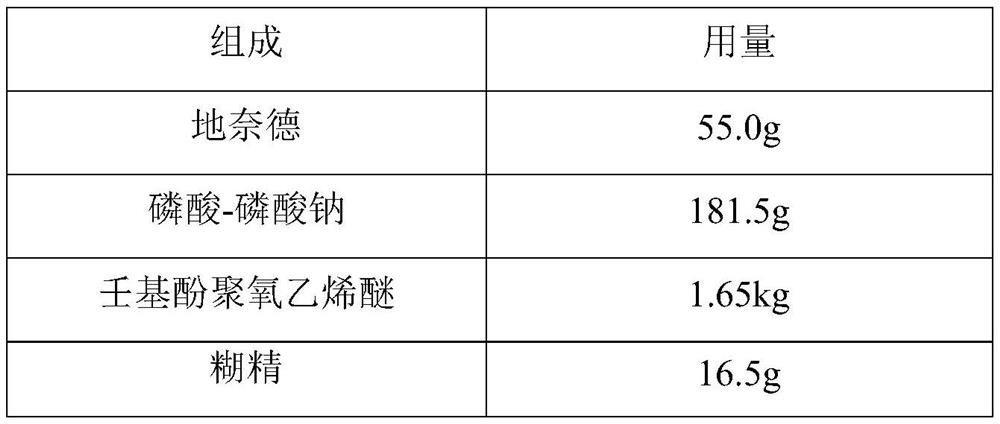
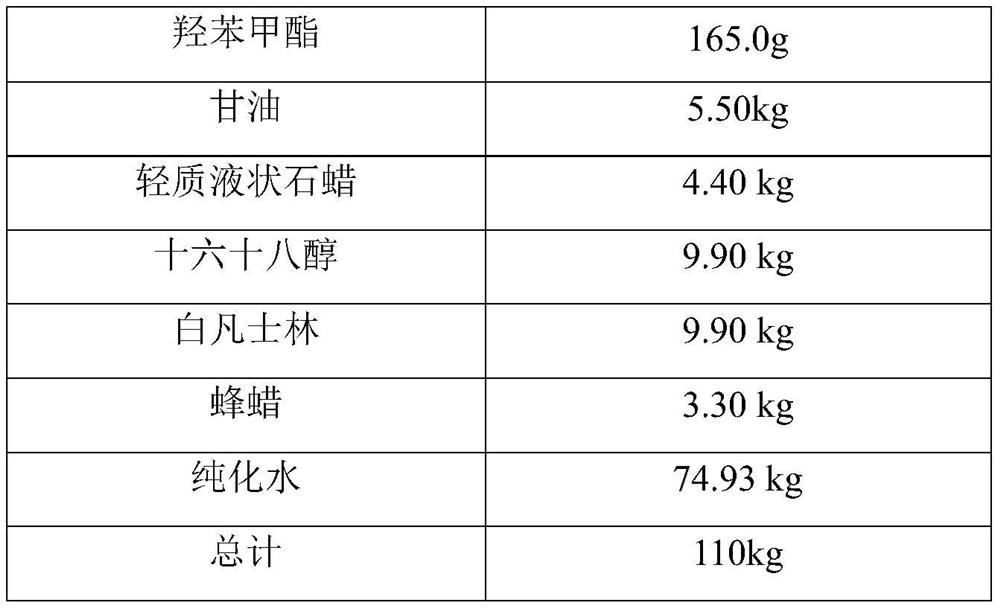
A kind of desonide cream and preparation method thereof
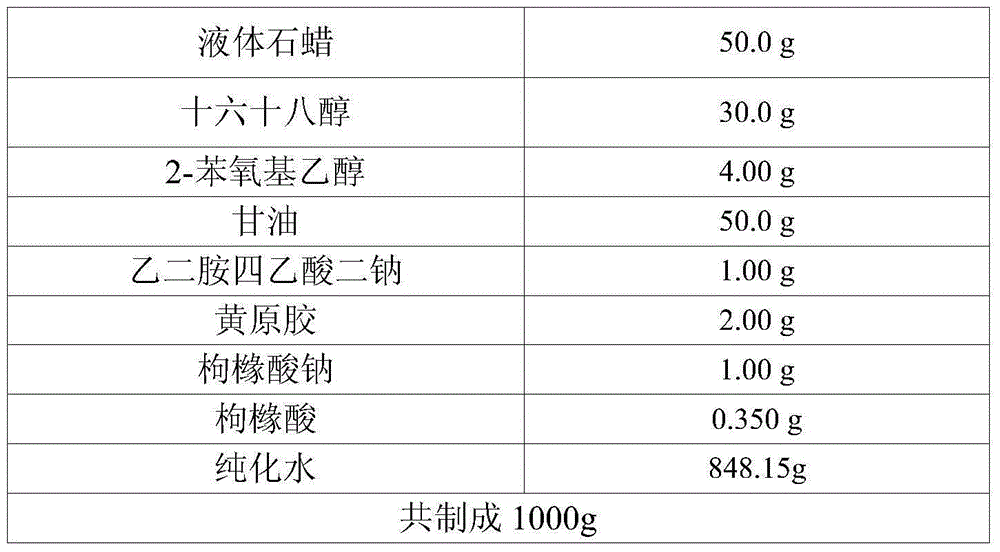
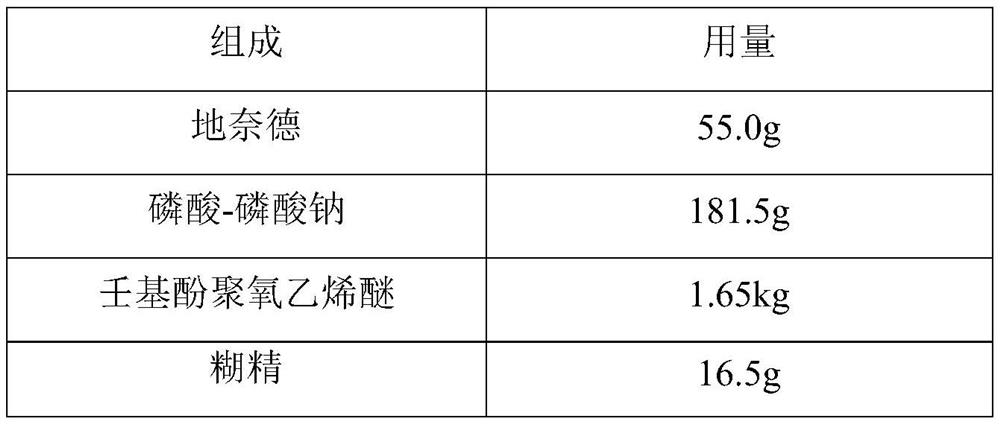
ActiveCN113413363BImprove stabilitySimple preparation processOrganic active ingredientsAntipyreticHigh humidityGlycerol
The invention provides a desonide cream and a preparation method thereof, comprising as active ingredients desonide, a polyoxyethylene ether emulsifier, a buffer pair, dextrin, glycerin, an antibacterial agent and an oily base; the buffer pair The pH range formed when dissolved in water is 4.5-5.5. The desonide cream has no obvious change in properties after high temperature and high humidity, low temperature, freezing and thawing and illumination, and the maximum single impurities and total impurities have no obvious increase, and can adapt to various environmental conditions encountered in storage and transportation. Meanwhile, the desonide cream disclosed by the invention has a simple preparation process and is easy to industrialize production.
Owner:FRONT PHARM PLC
Desonide cream and preparation method thereof
ActiveCN113413363AImprove stabilitySimple preparation processOrganic active ingredientsAntipyreticHigh humidityGlycerol
The invention provides desonide cream and a preparation method thereof. The desonide cream comprises desonide serving as an active component, a polyoxyethylene ether emulsifier, a buffer pair, dextrin, glycerol, an antibacterial agent and an oily matrix. The buffer pair is dissolved in water to achieve a pH value of 4.5-5.5. The desonide cream has no obvious change in character after being exposed to high temperature, high humidity, low temperature, freeze thawing and illumination, has no obvious increase in maximum single impurity and total impurity, and can adapt to various environmental conditions encountered in storage and transportation. Meanwhile, the desonide cream disclosed by the invention is simple in preparation process and easy for industrial production.
Owner:FRONT PHARM PLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com