Desonide cyclodextrin clathrate compound and method for preparing the same
A technology of cyclodextrin inclusion compound and cyclodextrin, which is applied in the field of cyclodextrin inclusion compound of desonide, can solve the problems of main drug content decrease, affect product stability, easy decomposition, etc., and achieve formulation stability Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 Preparation of desonide / β-cyclodextrin (molecular ratio: 2: 1) inclusion compound
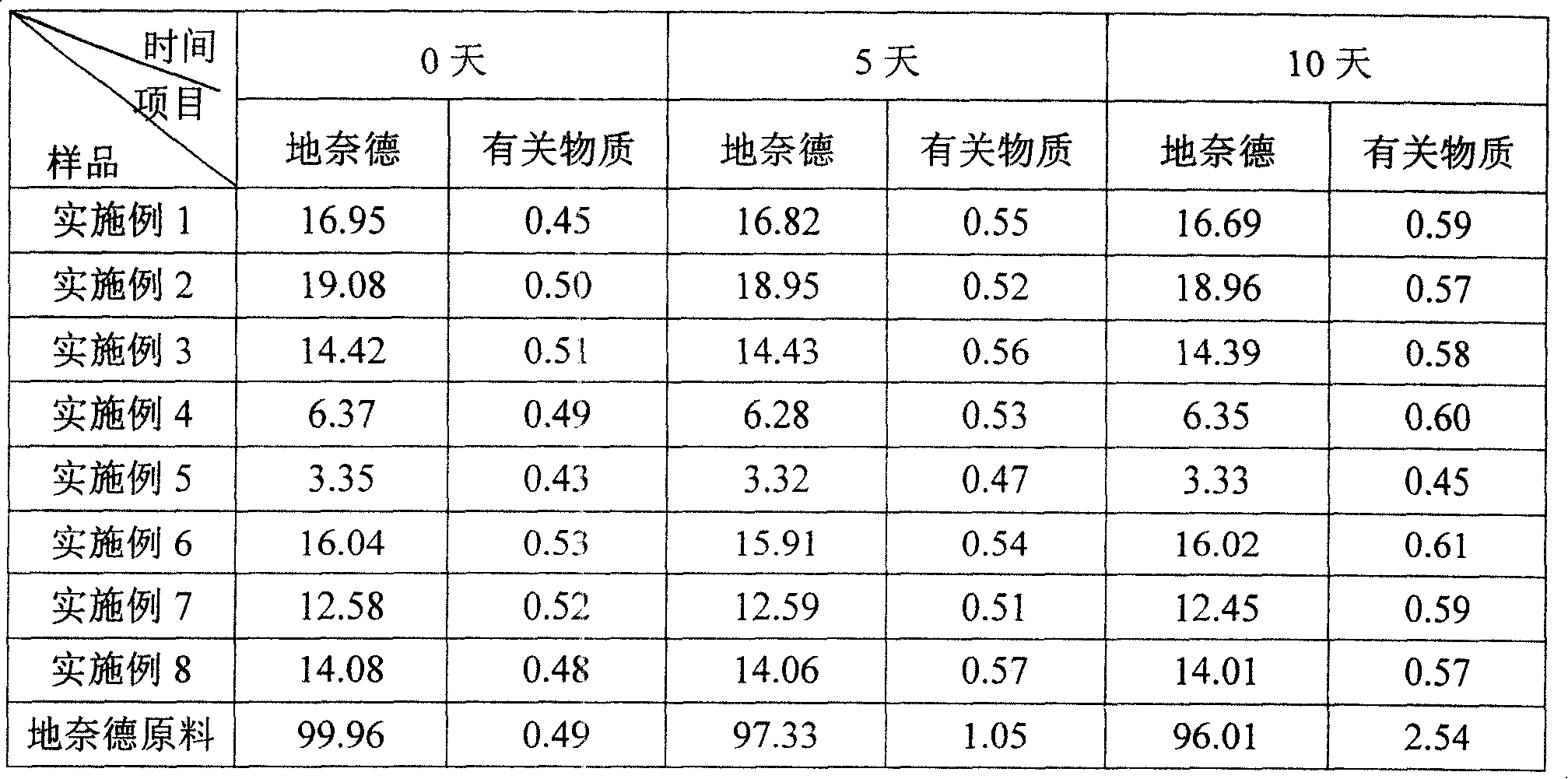
[0027] Add 1.36g of β-cyclodextrin (β-CD) into 73.5ml of water to make a saturated solution, dissolve and clarify under stirring; take another 1g of desonide raw material, dissolve it with 5ml of ethanol, slowly add β-CD under stirring to saturate In the solution, add ultrasonication for 60 minutes; stand still, filter the obtained precipitate, and wash with ethanol, and dry the obtained precipitate in an oven at 70°C for 6 hours to obtain cyclodextrin inclusion complex, which is a white solid in appearance. The content and encapsulation efficiency of the main drug in the inclusion compound are shown in Table 2.
[0028] Cyclodextrin inclusion complex detection
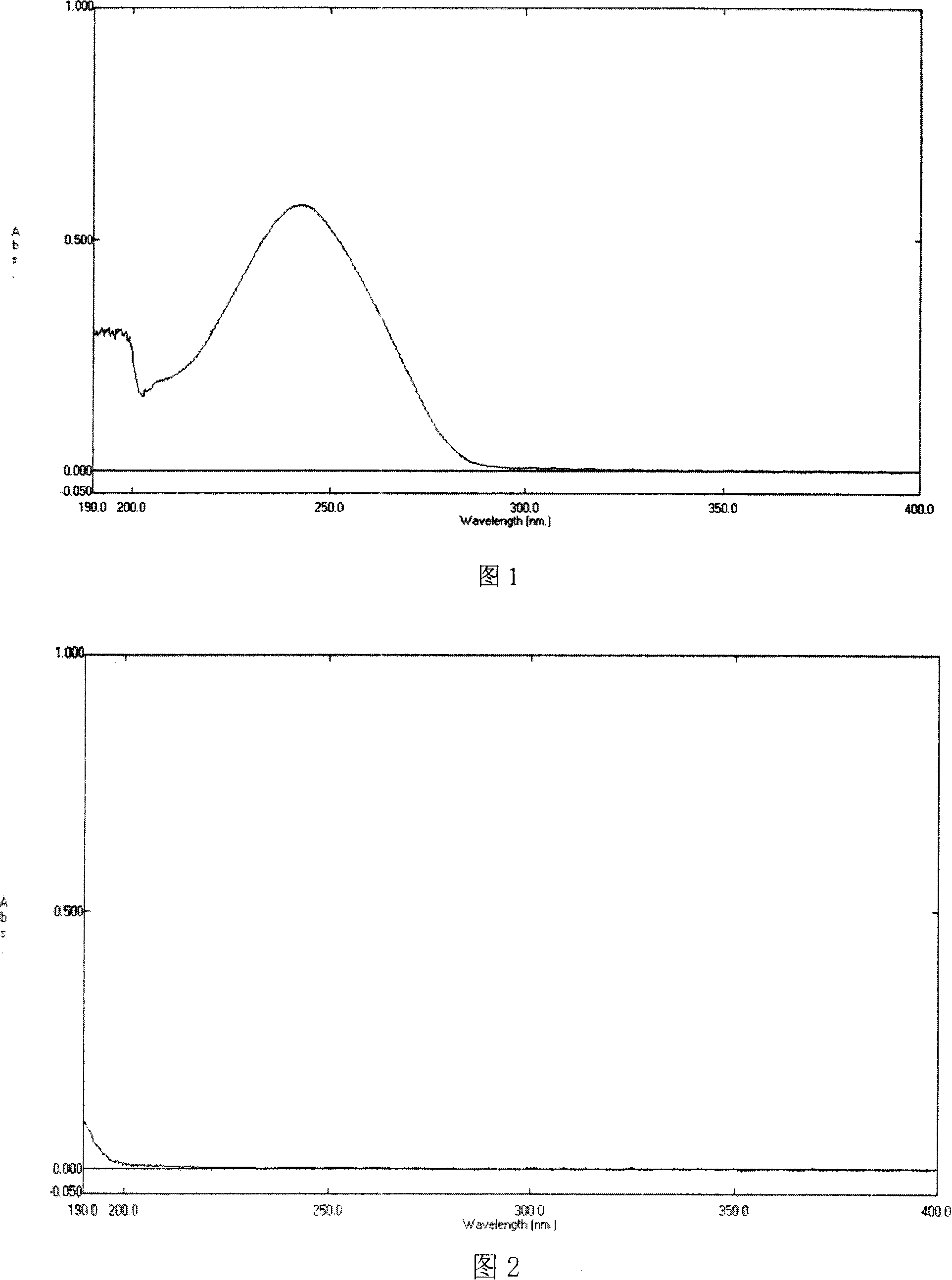
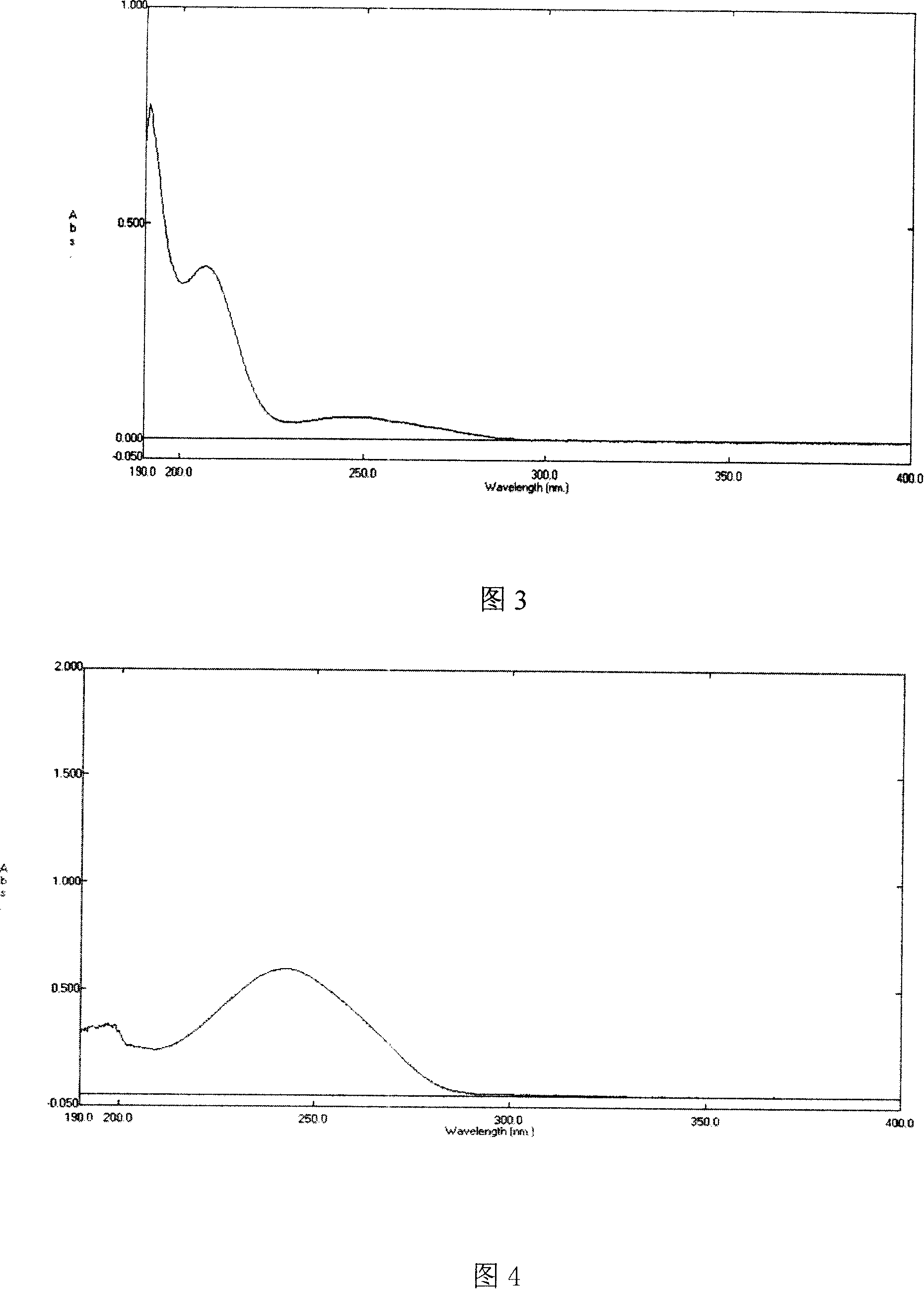
[0029] 1) UV absorption experiment
[0030] Scan the desonide solution with ultraviolet light to obtain the ultraviolet spectrum; calculate the content of desonide in the inclusion compound according to the conten...
Embodiment 2
[0036] Embodiment 2 Preparation of desonide / β-cyclodextrin (molecular ratio: 1: 1) inclusion compound
[0037] Add 2.72 g of D-cyclodextrin to 147 ml of water to form a saturated solution, and the rest are the same as in Example 1.
Embodiment 3
[0038] Example 3 Preparation of desonide / β-cyclodextrin inclusion compound (molecular ratio: 1:2)
[0039] Add 5.45 g of β-cyclodextrin and 295 ml of water to form a saturated solution, and the rest are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com